Definition of the term synthetic fuel code
The term "synthetic fuel" has several different meanings and can include different types of fuel. The traditional definition set by the International Energy Agency defines "synthetic fuels" as any liquid fuel derived from coal or natural gas. The US Energy Information Association defines synthetic fuels in its 2006 annual report as fuels derived from coal, natural gas, biomass or animal feed by chemical conversion into synthetic oil and/or synthetic liquid products. Numerous definitions of synthetic fuel include fuels produced from biomass as well as industrial and municipal waste. On the one hand, “synthetic” means that the fuel is produced artificially. Unlike synthetic fuel, conventional fuel is usually obtained by separating crude oil into separate fractions (distillation, rectification, etc.) without chemically modifying the components. However, various chemical processes can also be used in the production of traditional fuels. The term “synthetic” can emphasize, on the other hand, that the fuel was produced by chemical synthesis processes, that is, the production of higher-level compounds from several lower compounds. This definition applies in particular to XtL fuels, in which the feedstock is first decomposed into synthesis gas of lower compounds (H2, CO, etc.) in order to obtain higher hydrocarbons (Fischer-Tropsch synthesis). However, even with conventional fuels, chemical processes can be part of the production process. For example, hydrocarbons with carbon chains that are too long can be broken down into shorter chain products, such as those found in gasoline or diesel fuel, through so-called cracking. As a result, depending on the definition, it may not be possible to clearly distinguish conventional from synthetic fuels. Although there is no precise definition, the term "synthetic fuel" is generally limited to XtL fuel. The difference between synthetic and alternative fuels lies in the method of application of the fuel. That is, alternative fuels may require more extensive engine or fuel system modifications, or even the use of a non-traditional engine type (such as steam).
What makes it possible to ensure high heat transfer from smokeless briquettes? And how did you measure it?
Sergey Stepanov:
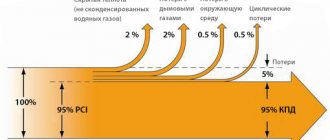
To measure heat transfer, 10 kilograms of briquettes were burned in a household boiler, and the supply of useful heat (hot water) was determined using a heat meter.
The high efficiency of the boiler is ensured by the fact that the boiler operates evenly most of the time. When burning coal, the combustion mode is different - first there is a peak heat release, then attenuation. Therefore, with coal, most of the heat simply flies away into the chimney.
By the way, when we talk about high heat transfer and fuel economy, it is important to understand that you need to load briquettes in the same volume as you usually load coal. The fact that you need 1.5-2 times less briquettes for heating means that you will have to load fuel 1.5-2 times less often
There is no need to reduce the single portion of loading!
Main coal products
The most conservative estimates indicate that there are 600 types of coal products. Scientists have developed various methods for obtaining processed coal products. The processing method depends on the desired end product. For example, to obtain clean products, the primary products of coal processing - coke oven gas, ammonia, toluene, benzene - use liquid washing oils. Special devices ensure sealing of products and protecting them from premature destruction. Primary processing processes also involve the coking method, in which coal is heated to a temperature of +1000°C with the access to oxygen completely blocked. Upon completion of all necessary procedures, any primary product is further purified. Main products of coal processing:
- naphthalene
- phenol
- hydrocarbon
- salicylic alcohol
- lead
- vanadium
- germanium
- zinc.
Without all these products, our life would be much more difficult. Take the cosmetics industry, for example, it is the most useful area for people to use coal processing products. A coal processing product such as zinc is widely used to treat oily skin and acne. Zinc and sulfur are added to creams, serums, masks, lotions and tonics. Sulfur eliminates existing inflammation, and zinc prevents the development of new inflammations. In addition, medicinal ointments based on lead and zinc are used to treat burns and injuries. An ideal assistant for psoriasis is the same zinc, as well as clay products of coal. Coal is the raw material for creating excellent sorbents, which are used in medicine to treat diseases of the intestines and stomach. Sorbents containing zinc are used to treat dandruff and oily seborrhea. As a result of a process such as hydrogenation, liquid fuel is produced from coal at enterprises. And the combustion products that remain after this process are ideal raw materials for a variety of building materials that have fire-resistant properties. For example, this is how ceramics are created.
| Direction of use | Brands, groups and subgroups |
| 1. Technological | |
| 1.1. Layer coking | All groups and subgroups of brands: DG, G, GZhO, GZh, Zh, KZh, K, KO, KSN, KS, OS, TS, SS |
| 1.2. Special preparation processes for coking | All coals used for layer coking, as well as grades T and D (DV subgroup) |
| 1.3. Production of generator gas in stationary gas generators: | |
| mixed gas | Brands KS, SS, groups: ZB, 1GZhO, subgroups - DGF, TSV, 1TV |
| water gas | Group 2T, as well as anthracites |
| 1.4. Production of synthetic liquid fuels | Brand GZh, groups: 1B, 2G, subgroups - 2BV, ZBV, DV, DGV, 1GV |
| 1.5. Semi-coking | Brand DG, groups: 1B, 1G, subgroups - 2BV, ZBV, DV |
| 1.6. Production of carbon filler (thermoanthracite) for electrode products and foundry coke | Groups 2L, ZA, subgroups - 2TF and 1AF |
| 1.7. Production of calcium carbide, electrocorundum | All anthracites, as well as subgroup 2TF |
| 2. Energy | |
| 2.1. Pulverized and layer combustion in stationary boiler plants | Weight of brown coals and atracites, as well as bituminous coals not used for coking. Anthracites are not used for flare-bed combustion |
| 2.2. Combustion in reverberatory furnaces | Brand DG, i group - 1G, 1SS, 2SS |
| 2.3. Combustion in mobile heating units and use for municipal and domestic needs | Grades D, DG, G, SS, T, A, brown coals, anthracites and hard coals not used for coking |
| 3. Production of building materials | |
| 3.1. Lime | Brands D, DG, SS, A, groups 2B and ZB; grades GZh, K and groups 2G, 2Zh not used for coking |
| 3.2. Cement | Brands B, DG, SS, TS, T, L, subgroup DV and grades KS, KSN, groups 27, 1GZhO not used for coking |
| 3.3. Brick | Coals not used for coking |
| 4. Other production | |
| 4.1. Carbon adsorbents | Subgroups: DV, 1GV, 1GZHOV, 2GZHOV |
| 4.2. Active carbons | Group ZSS, subgroup 2TF |
| 4.3. Ore agglomeration | Subgroups: 2TF, 1AV, 1AF, 2AV, ZAV |
Help with poisoning
It is important to know that the sooner help is provided for poisoning, the greater the effect that can be achieved. At the first signs of ill health, you need to take 6-8 tablets of activated carbon, washing them down with enough water. Crushed tablets can be mixed in a glass of water and drunk
Considering that charcoal does not dissolve in water, the resulting suspension must be shaken thoroughly before use.
The crushed tablets can be mixed in a glass of water and drunk. Considering that charcoal does not dissolve in water, the resulting suspension must be shaken thoroughly before use.
At the first signs of ill health, you need to take 6-8 tablets of activated carbon, washing them down with enough water. The crushed tablets can be mixed in a glass of water and drunk. Considering that charcoal does not dissolve in water, the resulting suspension must be shaken thoroughly before use.
The drug is continued until recovery, drinking 3-4 tablets at a time.
In case of acute intoxication, first cleanse the stomach with charcoal diluted in water (10-20 g of charcoal per 0.1 liter of water), and then give the patient 6-8 tablets.
Alcohol poisoning is treated according to the same scheme; the instructions for the drug recommend taking 3-5 tablets an hour or two before alcohol to reduce the damage done to the body.
In case of severe vomiting, you must first take antiemetic drugs, and only then take activated charcoal.
Coal
Processing of this type of raw material is carried out in three directions: hydrogenation, coking and incomplete combustion. Each of these types involves the use of a special technological process.
Coking involves keeping the raw material at a temperature of 1000-1200 o C, where there is no access to oxygen. This process allows for complex chemical transformations, which result in the formation of coke and volatile products. The first one, in a cooled state, is sent to metallurgy enterprises. The volatile products are cooled, after which coal tar is obtained. There are still many uncondensed substances remaining. If we talk about why oil is better than coal, it should be noted that much more finished products are obtained from the first type of raw material. Each of the substances is sent to a specific production.
At the moment, oil is even being produced from coal, which makes it possible to obtain much more valuable fuel.
Coal appeared on planet Earth about 360 million years ago. Scientists called this segment of our history the Carboniferous or Carboniferous period. At the same time, the appearance of the first terrestrial reptiles and the first large plants was recorded. Dead animals and plants decomposed, and a colossal amount of oxygen actively contributed to the acceleration of this process. Now on our planet there is only 20% oxygen, but at that time animals breathed deeply, because the amount of oxygen in the Carboniferous atmosphere reached 50%. It is this amount of oxygen that we owe to the modern wealth of coal deposits in the bowels of the Earth. But coal is not everything. Due to various types of processing, a huge number of different useful substances and products are obtained from coal. What is made from coal? This is exactly what we will talk about in this article.
What is obtained from coal
You, of course, know that coal is a fuel used both in everyday life and in industry. Coal was the first fossil material to be used as fuel. It was thanks to coal that the industrial revolution took place. In the 19th century, vehicles consumed a lot of coal. In 1960, world energy production was 50% dependent on coal. However, by 1970 its share had dropped to one third as oil and gas became more popular energy sources. However, the scope of coal use is not limited to this. Coal is a valuable raw material for the metallurgical and chemical industries. The coal industry provides coking of coal. Coke plants consume up to a quarter of the coal produced. Coking processes coal by heating it to 950-1050°C without oxygen. When coal decomposes, it forms a solid product - coke and a volatile product - coke oven gas. The mass of coke makes up 75-78% of the mass of processed coal. This material is used to smelt cast iron and is also used as fuel. The mass of coke oven gas is 25% of the mass of coal. The volatile products formed during coal coking are condensed with water vapor, resulting in the release of coal tar and tar water. The mass of coal tar is 3-4% of the mass of coal. This product is a complex mixture of organic substances. At the moment, only 60% of the components of coal tar have been identified, and this is more than 500 substances! The resin is used to produce naphthalene, anthracene, phenanthrene, phenols and coal oils. From the tar water (its mass is 9-12% of the mass of coal), pyridine bases, phenols, and ammonia are separated by steam distillation. Unsaturated compounds contained in crude benzene are used to produce coumaron resins, which are used to produce varnishes, paints, linoleum and rubber. Artificial graphite is also made from coal. Coal can serve as an inorganic raw material. During industrial processing, rare metals such as molybdenum, gallium, germanium, vanadium, lead, zinc, and sulfur are extracted from it. Waste obtained from coal mining and processing, as well as ash from coal combustion, is used in the production of refractory raw materials, ceramics, building materials, abrasives, and alumina. In total, processed coal can produce more than 400 different products, the cost of which is 20-25 times higher than the cost of the coal itself, while the cost of by-products obtained at coke plants exceeds the cost of the coke itself.
By the way... Coal is far from the best fuel. It has a serious drawback: when it is burned, large emissions are generated, both gaseous and solid (ash), which pollute the environment. In most developed countries, the level of emissions allowed when burning coal is strictly controlled by legislation. Various filters are used to reduce emissions.
Solid and gaseous fuels edit code
In some third world countries, firewood and charcoal are still the main fuel available to the population for heating and cooking (about half of the world's population lives this way). This in many cases leads to deforestation, which in turn leads to desertification and soil erosion. One of the ways to reduce the population's dependence on wood sources is to introduce technology for briquetting agricultural waste or household waste into fuel briquettes. Such briquettes are produced by pressing a slurry obtained by mixing waste with water on a simple lever press, followed by drying. This technology, however, is very labor-intensive and requires the availability of a source of cheap labor. A less primitive option for producing briquettes is to use hydraulic pressing machines.
Some gaseous fuels can be considered variants of synthetic fuels, although this definition may be controversial since engines using such fuels require significant modification. One of the widely discussed options for reducing the contribution of motor vehicles to the accumulation of carbon dioxide in the atmosphere is the use of hydrogen as a fuel. Hydrogen engines do not pollute the environment and emit only water vapor. Hydrogen-oxygen fuel cells use hydrogen to directly convert the energy of a chemical reaction into electrical energy. Since hydrogen is produced either by methods that require large amounts of electricity, or by the oxidation of hydrocarbon fuels, the environmental and, especially, economic advantages of such fuel are highly controversial.
Full article Hydrogen energy
.
Dimethyl etheredit | edit code
Dimethyl ether is obtained by dehydration of methanol at 300-400 °C and 2-3 MPa in the presence of heterogeneous catalysts - aluminosilicates. The degree of conversion of methanol into dimethyl ether is 60%, into zeolites - almost 100%. Dimethyl ether is an environmentally friendly fuel without sulfur content, and the emission of nitrogen oxides in exhaust gases is 90% less than that of gasoline. The cetane number of dimethyl diesel is more than 55, while that of classic petroleum diesel is from 38 to 53. The use of dimethyl ether does not require special filters, but it requires alteration of the power supply systems (installation of gas equipment, adjustment of mixture formation) and engine ignition. Without modification, it can be used on cars with LPG engines with a 30% methanol content in the fuel.
The calorific value of DME is about 30 MJ/kg, for classical petroleum fuels it is about 42 MJ/kg. One of the features of the use of DME is its higher oxidizing ability (due to the oxygen content) than that of classical fuel.
In July 2006, the National Development and Reform Commission (NDRC) (China) adopted a standard for the use of dimethyl ether as a fuel. The Chinese government will support the development of dimethyl ether as a possible alternative to diesel fuel. In the next 5 years, China plans to produce 5-10 million tons of dimethyl ether per year.
Cars with engines running on dimethyl ether are being developed by KAMAZ, Volvo, Nissan and the Chinese company Shanghai Automotive.
Obtaining biofuel
Biofuels can also be produced using algae, which are bred in artificial reservoirs. Agricultural crops do not grow on such soil. As algae grows, they increase their levels of fats and bio-oils through natural photosynthesis, making them similar to oil.
To grow algae, you need ultraviolet light, water, and carbon dioxide. When algae grows, they reduce greenhouse gases as they absorb carbon dioxide. Algae produce more biofuel than crops.
Today, several methods for producing biofuel are known. Biomass can be pieces of wood, straw, etc. They are used to make diesel fuel without sulfur and other impurities. Among other things, biodiesel, when burned, restores to the atmosphere the amount of carbon dioxide that plants absorbed during their growth.
During the processing of vegetable oil, in addition to fuel, glycerin and potassium sulfate are obtained. Biodiesel contains almost no sulfur and benzene. The decomposition of this fuel does not harm the environment, and there are fewer exhaust gases, unlike conventional diesel fuel. Vegetable fuel is highly flammable. When processing the oil, glycerin and sodium sulfate are obtained.
In the near future, it is planned to build a plant for processing sawdust and extracting pure biodiesel.
During the synthesis process, synthetic fuel is obtained from coal. Firewood releases after combustion, high humidity and without the required amount of oxygen. Fuel from wood waste does not emit carbon dioxide during combustion. There is no sulfur in synthetic diesel fuel.
Oil
If we further understand what is obtained from coal and oil, then it is worth mentioning the diesel fraction of oil refining, which usually serves as fuel for diesel engines. Fuel oil contains high-boiling hydrocarbons. Various lubricating oils are usually obtained from fuel oil through distillation under reduced pressure. The residue that exists after processing fuel oil is usually called tar. A substance such as bitumen is obtained from it. These products are intended for use in road construction. Fuel oil is also often used as boiler fuel.
Story
NYMEX West Texas Intermediate oil prices
During World War II, Germany largely, up to 50% in some years, met its fuel needs by creating production facilities for converting coal into liquid fuels. According to “Hitler’s personal architect” Albert Speer, Germany was technically defeated on May 12, 1944, when, as a result of massive Allied bombing, 90% of the factories producing synthetic fuel were destroyed.
Similarly, South Africa, with the same goals, created the company Sasol Limited, which during Apartheid helped the economy of this state to function successfully despite international sanctions.
In the US, producers of such fuels often receive government subsidies and therefore are sometimes made from a mixture of coal and biological waste. Such methods of obtaining government subsidies have been criticized by the “greens” as an example of abuse of the tax system by corporations. Synthetic diesel fuel, produced in Qatar from natural gas, has a low sulfur content and is therefore blended with conventional diesel fuel to reduce the sulfur level in the mixture, which is necessary for marketing diesel fuel in those US states where there are particularly high fuel quality requirements (for example in California).
| Synthetic liquid fuels and gas from solid fossil fuels are now produced on a limited scale. Further expansion of synthetic fuel production is hampered by its high cost, which significantly exceeds the cost of petroleum-based fuels. Therefore, new economical technical solutions in the field of synthetic fuel are now being intensively searched. The search is aimed at simplifying known processes, in particular, at reducing the pressure during coal liquefaction from 300-700 atmospheres to 100 atmospheres and below, increasing the productivity of gas generators for processing coal and oil shale, and also developing new catalysts for the synthesis of methanol and gasoline based on it. |
Currently, the use of Fischer-Tropsch technology is possible only at stable oil prices above $50-55 per barrel.
Making gasoline from old tires
You can make your own gasoline using old rubber tires.
For this you will need:
- rubber waste;
- bake;
- distiller;
- containers made of fireproof materials.
Step-by-step instructions for making gasoline from rubber tires are as follows:
- It is necessary to prepare a metal barrel with a tight-fitting lid. In addition, you will need a heat-resistant tube. It must be connected from above to the lid. This will create a homemade retort. Then you need a container for condensate and another small container with two tubes to create a water seal. One tube is lowered into the water, and the second is held above it.
- Next, you need to assemble a device for producing carbon in liquid form. To do this, we connect the tube from our retort to the condensate. Then we also connect the condensate and the water seal with a hose. We connect the second tube to the stove, on which we install the retort. The result is a closed-loop system for cracking at high temperatures.
- Place the rubber in the retort and close the lid tightly, then heat it over high heat. At high temperatures, rubber molecules are destroyed. Sublimation occurs, i.e., a transition from a solid to a gaseous state, bypassing the liquid stage. This gas then enters our condenser, where the temperature is much lower. The vapors condense, and as a result, we get oil in liquid form.
- The resulting substance must be purified; for this you will need a distiller, which is often used when using moonshine stills. The suspension is brought to a boil at a temperature of 200 degrees, and gasoline is obtained.
Ethers
Esters are colorless, mobile, low-boiling liquids with a characteristic odor. Methyl tert-butyl ether (MTBE) is currently considered the most promising anti-knock agent. In Russia it is allowed to add it to automobile fuels in amounts up to 15%. The limitations are caused by the characteristics of the performance characteristics: relatively low heat of combustion and high aggressiveness towards rubber. According to road test results, unleaded gasoline containing 7-8% MTBE outperforms leaded gasoline at all driving speeds. The addition of 10% MTBE to gasoline increases the octane number according to the research method by 2.1–5.9 units, and 20% by 4.6–12.6 units, and therefore it is more effective than such well-known additives as alkyl gasoline and methanol . The use of fuel with methyl tert-butyl ether somewhat improves the power and economic performance of the engine. MTBE is a colorless, transparent liquid with a pungent odor. The boiling point is 54-55°C, the density is 0.74 g/cm3. The octane number according to this method is 115-135 points. World production of MTBE amounts to tens of millions of tons per year.
As potential antiknock agents, it is possible to use ethyl tert-butyl ether, tert-amyl methyl ether, as well as methyl ethers obtained from C6-C7 olefins.
Properties of some esters.
| Ether | Formula | OCHIM | BSMM | OVSD | Boiling point, °С |
| MTBE | CH3-OC(CH3)3 | 118 | 110 | 114 | 55 |
| ETBE | C2H5-OC(CH3)3 | 118 | 102 | 110 | 70 |
| MTAE | CH3-OC(CH3)2C2H5 | 111 | 98 | 104,5 | 87 |
| DIPE | (CH3)2CH-O-CH(CH3)2 | 110 | 99 | 104,5 | 69 |
To obtain AI-95 and AI-98 gasolines, the additives MTBE or its mixture with tert-butyl alcohol, called Faterol - the trade name of Octane-115, are usually used. The disadvantage of such oxygen-containing components is the volatilization of esters in hot weather, which leads to a decrease in octane number.
Demonstration projects and commercial development
The 250-kilowatt methane synthesis plant was built by the Center for Solar Energy and Hydrogen Research (ZSW) in Baden-Württemberg and the Fraunhofer Society in Germany and began operating in 2010. It is being upgraded to 10 megawatts and is scheduled for completion in the fall. 2012
George Olah's carbon dioxide recycling plant, operated by Carbon Recycling International in Grindavik, Iceland, has been producing 2 million liters of methanol transport fuel per year from flue gases from the Svartsengi power station since 2008. Its capacity is 5 million liters per year. .
Audi has built a zero-carbon liquefied natural gas (LNG) plant in Werlte, Germany. The plant is designed to produce transportation fuel to offset the CNG used in their cars, and can hold 2,800 metric tons of CO2 per year at its original capacity.
Commercial development is underway in Columbia, South Carolina, Camarillo, California, and Darlington, England. A demonstration project in Berkeley, California, proposes the synthesis of fuels and edible oils from recovered flue gases.
Liquid fuel from gases
It is difficult to imagine that from such simple substances as carbon monoxide (that is, carbon monoxide) and hydrogen, complex organic compounds and a wide variety of liquid fuels can be obtained.
To obtain liquid fuel, you need to have a mixture of these gases, in which for every part of carbon monoxide there would be two parts of hydrogen. This mixture is obtained in special devices - gas generators. A mixture of water vapor and air is blown through a layer of hot coke. Oxygen in the air combines with carbon to form carbon monoxide. This process is called coal gasification. When water molecules decompose, hydrogen is released. A mixture of hydrogen and carbon monoxide is sent to refrigerators. From here the so-called water gas goes into the reactor. At a temperature of 200°, under the influence of the most active catalysts - cobalt or nickel - carbon monoxide and hydrogen enter into a chemical compound. Complex heavy substances are formed from a large number of light gas molecules.
Catalysts not only contribute to the formation of simple compounds of carbon and hydrogen, but also influence further complexity - the polymerization of molecules: carbon atoms are connected in chains, rings, and are overgrown with hydrogen atoms. A wide variety of hydrocarbons re-emerge - from light gases (starting from methane) to solid, high-melting paraffins, containing up to 100 carbon atoms in each molecule. Approximately 60% of the initially taken gas mixture turns into liquid fuel. This is artificially prepared oil, not much different from ordinary, natural oil.
Let's enter the workshop where fuel synthesis takes place. The iron apparatus is surrounded by complex interweavings of thick pipes. The workshop is quiet and deserted. Special devices automatically control the process and record temperature and pressure themselves. It is interesting that the process of formation of liquid fuel occurs at normal atmospheric pressure and a temperature of only about 200°. When synthesizing fuel from gases, expensive equipment is not needed to create high pressures and temperatures. This distinguishes synthesis favorably from coal hydrogenation.
Soviet industry now produces hundreds of thousands of diesel engines running on mixtures of high-boiling heavy oil fuel.
There are more and more powerful 25-ton trucks - dump trucks, motor ships, excavators and other machines with diesel engines installed. The car and tractor fleet is increasing.
The production of artificial diesel fuel is also continuously growing.
This is how chemists manage processes to obtain the right grade of fuel.
The advantages of this method open up great prospects for it. Liquid fuel can be obtained from any, even the lowest-grade brown coal.
Preliminary gasification of fuel makes it possible to obtain gasoline from oil shale and even peat, not to mention the use of natural gas for this purpose. In 1951 - 1955, new plants were built to produce synthetic liquid fuel from coal, shale and peat. In the Estonian SSR alone, based on local shale, the production of such fuel will increase by 80% over the five-year period.
S. Gushchev Fig. B, Dashkov and A. Katkovsky magazine “Technology for Youth” No. 7, 1954
conclusions
Despite the fact that the separation of motor fuel from hard and brown coal is quite real and has long been tested in production, it is hardly possible to organize it at home. Of course, there will always be a few craftsmen - enthusiasts who love to achieve their goals and will be able to synthesize gasoline with their own hands. But to do this, you need to study the technology in detail and tinker a lot with the equipment, not to mention the fire hazard.
For a wide range of homeowners and car enthusiasts, obtaining diesel fuel and gasoline from coal is not available. And if you approach the issue from an economic point of view, then it is unprofitable. At the moment, until new inventions and developments have appeared on this topic, it is easier and more reliable to use regular, “petroleum” gasoline.
Better than in nature
Back at the end of the last century N.D
Zelinsky drew attention to the difference in the structure of oil molecules. Most of the molecules of high-quality Baku oil are closed rings of carbon atoms, to which hydrogen atoms are attached on the sides
The high quality of fuel primarily depends on this cyclic structure of molecules. Grozny oil contains less naphthenes - cyclic hydrocarbons. It is dominated by molecules of the methane series, stretched in the form of chains of atoms. Gasoline obtained from Grozny oil, when compressed in the engine cylinders, detonated and spontaneously exploded much earlier than the moment when the ignition spark jumped between the electrodes of the spark plug.
This phenomenon caused a lot of trouble for chemists and engine builders, who always sought to increase engine power. The power and efficiency of an engine depends primarily on how strongly the pistons in the cylinder compress the combustible mixture. The compression ratio (that is, the ratio of the volume of the entire cylinder to the volume of the combustible mixture extremely compressed in the cylinder) is one of the most important characteristics of the engine. The higher the compression ratio, the more powerful and economical the engine. If, for example, you increase the compression ratio of a car engine from 5.25 to 10.3, then the car, moving at a speed of 40 km/h, will consume half as much fuel and will travel twice as far on one tank of gasoline.
But here's the problem: the vapors of regular gasoline cannot withstand high compression and detonate. The engine quickly overheats and begins to knock, as if it is about to fall apart. Its power drops sharply.
During detonations, the piston rings and piston crown burn out, and the bearings are destroyed.
These fuel properties are assessed by the so-called octane number. If they say that the octane number of a fuel is 60, this means that its detonation properties are the same as those of a mixture containing 60% isooctane and 40% heptane. These two substances were not taken as a standard by chance: isooctane resists detonation very well (its octane number was therefore set to 100), and heptane, on the contrary, detonates more easily than all other liquid hydrocarbons (its octane number was taken to be 0).
The result is a kind of scale by which you can find out how it detonates and whether this or that type of gasoline is of high quality.
The higher the octane number of gasoline, the stronger the combustible mixture can be compressed in the cylinders without fear of detonation, the more powerful and economical the engine. At first, aircraft engines ran on gasoline with an octane rating of 50-55. The use of 87 octane gasoline in aviation made it possible to increase engine power by 30-35%, and the advent of 100-octane gasoline helped increase engine power by another 15-30%. In other words, modern engines have become almost twice as powerful as “old” engines with the same cylinder capacity.
It would seem that the quality of 100-octane gasoline is the limit set by nature itself. But this limit, like many others, was managed to be crossed by science, armed with advanced technology. Modern airplanes fly on gasoline with an octane rating much higher than 100. There is no oil in the world that contains gasoline of such high quality. Such gasoline can only be obtained artificially - through synthesis.
The synthesis of hydrocarbons has long been a tempting goal for many generations of chemists. Academician N.D. Zelinsky wrote in 1931: “When a chemist gets acquainted with the structure of petroleum hydrocarbons and studies their properties, he cannot help but be surprised at how easily nature has created these amazing forms, which are so difficult to prepare synthetically.”
Nowadays, high-quality liquid fuels are obtained from low-quality gasolines and gases by rearranging straight chains into branched and ringed structures.
Areas of application of activated carbon in modern life
The scope of application of activated carbon is very wide. Its properties were known back in ancient times - in Rus' it was made at home, most often from birch logs; for this you didn’t even need to do anything - just the coals left after lighting the bath were brought into the steam room for activation. In terms of quality, this prototype could not be compared with modern brands of coal, but even then it was used to treat gastric disorders in both people and livestock, it was used to filter water and homemade alcoholic drinks, and much more.
It was first used on an industrial scale by the military. Activated carbon became the key element of the gas mask developed by N.D. Zelinsky during the First World War - when German troops began releasing chlorine on the battlefield. Coal saved many lives in those years due to its absorbent properties.
In the modern world it is used in many areas:
- In the food industry (for example, for sugar purification)
- In the chemical industry as a reaction catalyst
- In medicine
- In pharmaceuticals
- In purification facilities for purifying air and water from industrial waste
- In household filters for drinking water
as well as in many other areas.
It's all about its properties - activated carbon is an excellent absorbent, very light, very effective, and most importantly - very cheap. It can be produced almost anywhere; the production technology, although not simple, does not require a long time and overly complex processes - only two devices can handle everything. The site elgreloo.com invites you to familiarize yourself with the technology for manufacturing activated carbon.
Processing waste into fuel in Russia
In January 2022, the country's President V. Putin signed a decree on the creation, which will become the country's unified garbage operator in the form of a public company (PPC); The functions of the founder will be performed by the Ministry of Natural Resources. The operator will deal with state programs for waste management and attract investors for waste disposal projects.
Innovation
Waste processing complexes: For the first time, within the framework of domestic research, the task was set (2011) to combine disparate advanced developments
across many industries.
Several options for environmentally friendly, high-tech waste processing complexes that are competitive on the world market will be developed. Optimization of raw materials, heat, and gas flows
will ensure maximum production of liquid fuel fractions and building materials - without any process waste, except for waste catalytically purified gases. As a result of processing, profitable products will be produced: fuel, additives, construction materials.
At the 1st stage, it is planned to complete the experimental line for research, testing, certification and patenting. This work will be carried out jointly with the Skolkovo Foundation, of which .
It is planned to build mobile or stationary processing complexes
consisting of 1-5 lines of the same type with an annual processing volume of 50-250 thousand tons of prepared solid waste (newly generated and landfill), sorting “tailings”, sludge sediments, peat, coal sludge, wood waste and other organics. As a result of processing, the following commercial products will be produced:
- diesel fuel
- chemical products: (benzene, toluene and nefras or combined BTK fraction),
- cement,
- gas foam concrete.
see also
- Alternative automobile fuel
- Synthetic natural gas
- The methanol economy is a hypothetical future energy economy in which fossil fuels are replaced by methanol.
- Dry distillation
- GTL (Gas-to-liquids) is the process of converting natural gas into high-quality, sulfur-free motor fuels and other (heavier) hydrocarbon products.
- Hydrolysis production
- Biofuel
- Global Energy
- Solar oven is a simple device for using sunlight to cook food without using fuel or electricity.