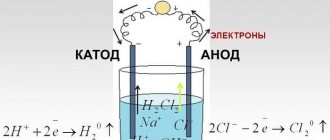
Oil refineries produce fuel. But the cost in liters at gas stations is so daunting that car owners often ask how they can make gasoline at home. Every year, heaps of waste are released into the atmosphere. And if you put them to work, select a unit for distillation, then you can completely try to make methanol (methyl alcohol) on your own.
What is methanol and how to make it
Methanol is a poisonous colorless solvent with the taste of drinking alcohol, the octane number of which is 150. Essentially, it is the same fuel. Although it differs from motor gasoline in that after filling:
- Increases engine power by 20%, service life several times
- Does not emit harmful components when the engine is running. This means it is an environmentally friendly product.
How to make gasoline from methanol in artisanal conditions? It is possible using moonshine or denaturation technology (adding naphtha, kerosene). The process of methanol distillation is a step-by-step process, which differs from the distillation of moonshine. The output should be clean fuel with the least amount of water. During production, it will be necessary to install cleaning filters to the unit that will remove excess liquid from fuel alcohol.
Ethanol is a solvent. It will wash corroded dirt from the fuel lines into the cylinders. This means that the filters will serve as a separator of debris and water from the fuel tank. Step-by-step steps for making homemade gasoline from methanol:
- Selection of initial raw materials for making mash (wheat, corn, millet, Jerusalem artichoke).
- Combining foods with sugar to begin the fermentation process, then fermentation to produce alcohol.
- Selection of a unit made of stainless steel or iron. To obtain 3 liters of gasoline in 1 hour, it is enough to select thin copper tubes of the following dimensions: width - 30 cm, length - 50 cm, height - 20 cm, diameter - 75 mm. A capillary tube from a used refrigerator is suitable as a faucet, and a pressure reducing valve is from a gas cylinder.
- Installation of a mixer with a reactor horizontally for heating.
- Connecting the structure to a water supply system divided into 2 streams. One will go into the refrigerator through a faucet and hole. Another is to enter the faucet through a faucet with a hole.
Water will flow through the hole, begin to cool, and turn into condensate and synthesis gas. Natural gas connected to the pipeline goes into the mixer, mixes with water steam, heats up to t +120 degrees. using a torch
It is important to adjust the faucet immediately. This will maintain optimal pressure in the condenser. You can't close it completely. It is enough to open it slightly for water to start flowing. When the temperature in the reactor reaches +250, you should open the tap a little more so that gasoline flows out in a thin stream. Still open slightly in case of leakage of fuel with gas impurities.
The unit is connected to a gas burner and adjusted to high performance. It is important that the least amount of steam is formed in the mixer, and that there is practically no water left in the fuel at the outlet. You can check the content with an alcohol meter. A pressure gauge is attached to the hole on the condenser to keep the pressure under control within 10 atm.
Tap water contains chlorine. This means that it will instantly lead to poisoning of the catalyst of the second reactor. That is why many craftsmen pour distilled water into the installation (reactor). The gas also contains sulfur impurities and active organic compounds. To achieve better results, it is better to use monoethanolamine gas purification.
Ethers
Esters are colorless, mobile, low-boiling liquids with a characteristic odor. Methyl tert-butyl ether (MTBE) is currently considered the most promising anti-knock agent. In Russia it is allowed to add it to automobile fuels in amounts up to 15%. The limitations are caused by the characteristics of the performance characteristics: relatively low heat of combustion and high aggressiveness towards rubber. According to road test results, unleaded gasoline containing 7-8% MTBE outperforms leaded gasoline at all driving speeds. The addition of 10% MTBE to gasoline increases the octane number according to the research method by 2.1–5.9 units, and 20% by 4.6–12.6 units, and therefore it is more effective than such well-known additives as alkyl gasoline and methanol . The use of fuel with methyl tert-butyl ether somewhat improves the power and economic performance of the engine. MTBE is a colorless, transparent liquid with a pungent odor. The boiling point is 54-55°C, the density is 0.74 g/cm3. The octane number according to this method is 115-135 points. World production of MTBE amounts to tens of millions of tons per year.
As potential antiknock agents, it is possible to use ethyl tert-butyl ether, tert-amyl methyl ether, as well as methyl ethers obtained from C6-C7 olefins.
Properties of some esters.
| Ether | Formula | OCHIM | BSMM | OVSD | Boiling point, °С |
| MTBE | CH3-OC(CH3)3 | 118 | 110 | 114 | 55 |
| ETBE | C2H5-OC(CH3)3 | 118 | 102 | 110 | 70 |
| MTAE | CH3-OC(CH3)2C2H5 | 111 | 98 | 104,5 | 87 |
| DIPE | (CH3)2CH-O-CH(CH3)2 | 110 | 99 | 104,5 | 69 |
To obtain AI-95 and AI-98 gasolines, the additives MTBE or its mixture with tert-butyl alcohol, called Faterol - the trade name of Octane-115, are usually used. The disadvantage of such oxygen-containing components is the volatilization of esters in hot weather, which leads to a decrease in octane number.
Homemade gasoline options
Producing real fuel is a complex, costly process. First, oil is extracted and then redirected for processing. It is hardly possible to produce high-quality gasoline at home. However, there is enough fuel for heating or refueling a chainsaw.
Petroleum products are found everywhere. This is garbage under people's feet (synthetic fabrics, polyethylene, plastic, rubber tires, rotten wood). From them, diesel fuel can be extracted for internal combustion engines if it is subjected to pyrolysis or heating under oxygen-free conditions. Recyclable materials will release carbon dioxide when burned. This means it will not harm the external environment. The output will be fuel no worse than gasoline from a gas station.
1 kg of bottle plastic yields 1 liter of fuel. You can make homemade gasoline in artisanal conditions from coal by heat treatment, old tires, rubber waste, crude oil and gasification. Some craftsmen have learned to extract gasoline for internal combustion engines. Good synthetic diesel fuel is produced by distilling plastic bottles and rubber tires.
Notes[edit
- It is more efficient, although time-consuming, to obtain charcoal from wood instead of using pure wood as fuel for the stove. But if you craft 4 boards from it, then each of them will also be able to smelt 1.5 units of raw materials, and in total they will burn for 60 seconds and smelt 6 units of raw materials. However, from one block of wood, by firing, you can get one piece of charcoal, which can already melt 8 things.
Thus, it is more efficient to take 7 blocks of wood, one of which is broken into planks, which are used to burn the remaining 6 blocks of wood into 6 units of charcoal. These 6 pieces of charcoal can smelt 48 items. Typically 7 blocks of wood can smelt 42 items as they make 28 planks. Thus, this process is more efficient than using wood as fuel. However, it takes more time, which is spent on creating charcoal and then directly smelting the desired item.
Making gasoline from coal
European countries, not having oil reserves, have long started producing gasoline from raw coal. Also in the pre-war years, Germany produced fuel thanks to the Ruhr coal basin with its large deposits of brown coal
How gasoline is separated from coal
Oil and coal have identical chemical compositions (hydrogen, carbon compounds), which can simply be replaced. This means that as the number of hydrogen molecules in coal increases or hydrogenates, oil will begin to be released first, then gasoline will be released through processing.
The separation of fuel from oil is possible in two ways: Fischer-Tropsch, Bergius by synthesis and gasification of fuel or liquefaction (hydrogenation).
Oil
If we further understand what is obtained from coal and oil, then it is worth mentioning the diesel fraction of oil refining, which usually serves as fuel for diesel engines. Fuel oil contains high-boiling hydrocarbons. Various lubricating oils are usually obtained from fuel oil through distillation under reduced pressure. The residue that exists after processing fuel oil is usually called tar. A substance such as bitumen is obtained from it. These products are intended for use in road construction. Fuel oil is also often used as boiler fuel.
What is hydrogenation
Hydrogenation is a technological process for producing synthetic gasoline from brown coal. The stages are as follows:
- softening coal, mixing with a fatty viscous liquid (fuel oil, tire oil) to obtain a paste-like component;
- placing the paste in an airtight container;
- adding solvent and catalyst to enrich coal.
Under the influence of a temperature of +500 degrees, high pressure of 200 atm, coal turns into a liquid state, then into a vapor state. It is spun in a centrifuge to remove coke and undergoes a distillation and hydrogenation process to obtain the final product.
Obtaining raw materials for biodiesel production at home
The great thing about biodiesel is that you can make it from a huge range of vegetable oils or animal fats (you could even theoretically get some free stuff from local restaurants). The process of obtaining raw materials is quite simple, like one, two, three. Contact local restaurants, find out if they have waste vegetable oils, and then find a way to transport this waste home. Ready!
Without a ready source of waste cooking oil, obtaining this raw material to create your own biodiesel becomes more difficult. Buying oil in stores to add to diesel fuel (diesel fuel) is expensive.
Another option is to create your own vegetable oil. The process is lengthy and impractical. Maybe in some distant hypothetical or post-apocalyptic future, when all other resources are exhausted, this will be economically feasible, but not now and not in our time.
Ten of the Strangest Energy Sources for Car Engines
Bottom line: With the proper knowledge of technology and technical means, it is somewhat easier to make this ethyl alcohol for cars than the same biodiesel fuel. However, without using the grown material for processing, such creation of home fuel turns into an expensive pleasure. We need to remember this.
Producing gasoline by gasification
The Fischer-Tropsch or gasification method is step-by-step:
- Coal raw materials are combined with water. Placed in a sealed steam vessel.
- Heats up to t + 350 degrees and is subjected to pressure of 30 atmospheres.
The process produces synthetic gas, which is placed in another sealed vessel filled with a catalyst (iron, cobalt). The fuel comes out and is cracked to produce diesel fuel.
Gasoline can be produced by thermal treatment of coal. Although it is difficult to produce in artisanal conditions. You will need equipment similar to a blast furnace. The method is identical to the pyrolysis process. Coal is placed in a vessel without oxygen, subjected to temperature +200 degrees and a decomposition process with a transition from a solid to a gaseous state.
Application
Production and use of synthesis gas
Ammonia production
The most important application of synthesis gas is the production of ammonia – NH3.
A mixture of nitrogen and hydrogen is passed over an iron catalyst (which contains alumina as a promoter). The reaction takes place under very harsh conditions - at a temperature of 420 C and a pressure of 280 atm.
Most ammonia production plants are equipped to separate CO from the feedstock, since CO can poison the catalyst. Most often, for this purpose, CO is treated with water vapor to produce CO and H2. Carbon dioxide is removed by extraction with ethanolamine, and H2 is recycled.
Methanol production
Methanol is often called wood alcohol. This is due to the fact that the first industrial method for producing methanol was the dry distillation of freshly cut wood from deciduous trees. In addition to other compounds, methanol is present in the volatile fraction. This is where the name “wood alcohol” comes from.
Since 1923, methanol has been produced industrially from synthesis gas; Currently, most methanol is obtained using this method. For several reasons, these plants are usually built near or even combined with ammonia production plants.
By the way, read this article too: What kind of oil to pour into the rotator
The technology and equipment are similar, and CO2 generated during ammonia synthesis can be used in the production of methanol. In this case, CO2 is reacted with methane and steam over a nickel catalyst, resulting in the formation of additional CO and H2, which then react to produce methanol.
The process is carried out at a pressure of 67 – 100 atm. And temperatures of 200 – 260°C on catalysts based on copper and zinc oxides.
Fischer-Tropsch synthesis
The catalytic hydrogenation of carbon monoxide (Fischer-Tropsch synthesis) to produce hydrocarbons, in particular paraffins, mainly of normal structure and olefins, is a heterogeneous reaction. It is carried out mainly over cobalt or iron catalysts using the purest possible mixtures of carbon monoxide and hydrogen. Optimal operating temperatures for synthesis vary for different catalysts.
Nickel and cobalt catalysts give optimal results at 170-205, iron - at 200-325°C. Synthesis on nickel catalysts is carried out practically only at normal pressure, since at elevated pressure the formation of carbonyls sharply increases.
Iron and cobalt catalysts can be used without the formation of carbonyls at pressures up to 20 atm.
It is believed that during the Fischer-Tropsch synthesis on iron catalysts, carbon monoxide is hydrogenated to form a methylene group, which then polymerizes. Carbon monoxide is converted to carbon dioxide. In this case, the oxygen of carbon monoxide binds, forming water. These reactions are favored by the higher synthesis temperature on iron catalysts and the activity of iron towards the conversion reaction.
By the way, read this article too: Aromatic hydrocarbons (Arenas)
From a technical point of view, the decisive factors in the Fischer-Tropsch synthesis are, firstly, the very high heat of reaction of the catalytic hydrogenation of carbon monoxide and, secondly, the need to very accurately maintain a constant synthesis temperature. Otherwise, the unwanted formation of methane increases significantly. In addition, at high temperatures, carbon deposition on the catalyst is observed, leading to its rapid deactivation.
How to make gasoline from gas
You will need a unit like a mixing vessel to understand how to make fuel from gas. For example, made of stainless steel and metal. Into the vessel:
- Water and propane-butane gas are added.
- Heating occurs to t inside the mixer +120, where mixing of water vapor with gas begins to occur.
Gas already in mixed form:
- fed into a reactor filled with a catalyst made of aluminum and nickel shavings;
- heats up to t+500 g, forming a synthetic substance;
- goes into the refrigerator, cools to t + 35-40 g;
- fed into a sealed container with a catalyst made of zinc and copper shavings;
- is subjected to pressure and t +270 degrees, forming synthetic fuel.
The resulting vapors are transferred from the container to the refrigerator. Subject to cooling and condensation under the influence of condensate. Gas and synthetic gasoline not dissolved in water enter the condenser. A synthetic product is drained from it. The gas can be sent back for recycling.
Better than in nature
Back at the end of the last century N.D
Zelinsky drew attention to the difference in the structure of oil molecules. Most of the molecules of high-quality Baku oil are closed rings of carbon atoms, to which hydrogen atoms are attached on the sides
The high quality of fuel primarily depends on this cyclic structure of molecules. Grozny oil contains less naphthenes - cyclic hydrocarbons. It is dominated by molecules of the methane series, stretched in the form of chains of atoms. Gasoline obtained from Grozny oil, when compressed in the engine cylinders, detonated and spontaneously exploded much earlier than the moment when the ignition spark jumped between the electrodes of the spark plug.
This phenomenon caused a lot of trouble for chemists and engine builders, who always sought to increase engine power. The power and efficiency of an engine depends primarily on how strongly the pistons in the cylinder compress the combustible mixture. The compression ratio (that is, the ratio of the volume of the entire cylinder to the volume of the combustible mixture extremely compressed in the cylinder) is one of the most important characteristics of the engine. The higher the compression ratio, the more powerful and economical the engine. If, for example, you increase the compression ratio of a car engine from 5.25 to 10.3, then the car, moving at a speed of 40 km/h, will consume half as much fuel and will travel twice as far on one tank of gasoline.
But here's the problem: the vapors of regular gasoline cannot withstand high compression and detonate. The engine quickly overheats and begins to knock, as if it is about to fall apart. Its power drops sharply.
During detonations, the piston rings and piston crown burn out, and the bearings are destroyed.
These fuel properties are assessed by the so-called octane number. If they say that the octane number of a fuel is 60, this means that its detonation properties are the same as those of a mixture containing 60% isooctane and 40% heptane. These two substances were not taken as a standard by chance: isooctane resists detonation very well (its octane number was therefore set to 100), and heptane, on the contrary, detonates more easily than all other liquid hydrocarbons (its octane number was taken to be 0).
The result is a kind of scale by which you can find out how it detonates and whether this or that type of gasoline is of high quality.
The higher the octane number of gasoline, the stronger the combustible mixture can be compressed in the cylinders without fear of detonation, the more powerful and economical the engine. At first, aircraft engines ran on gasoline with an octane rating of 50-55. The use of 87 octane gasoline in aviation made it possible to increase engine power by 30-35%, and the advent of 100-octane gasoline helped increase engine power by another 15-30%. In other words, modern engines have become almost twice as powerful as “old” engines with the same cylinder capacity.
It would seem that the quality of 100-octane gasoline is the limit set by nature itself. But this limit, like many others, was managed to be crossed by science, armed with advanced technology. Modern airplanes fly on gasoline with an octane rating much higher than 100. There is no oil in the world that contains gasoline of such high quality. Such gasoline can only be obtained artificially - through synthesis.
The synthesis of hydrocarbons has long been a tempting goal for many generations of chemists. Academician N.D. Zelinsky wrote in 1931: “When a chemist gets acquainted with the structure of petroleum hydrocarbons and studies their properties, he cannot help but be surprised at how easily nature has created these amazing forms, which are so difficult to prepare synthetically.”
Nowadays, high-quality liquid fuels are obtained from low-quality gasolines and gases by rearranging straight chains into branched and ringed structures.
Making gasoline from tires
Extracting fuel from rubber tires is profitable and exciting. You will need 3 metal barrels with lids, a blast furnace (heat source), a distiller, raw materials (waste).
Stages:
- cut the rubber into small pieces;
- take a fireproof container, connect a heat-resistant tube, immerse the prepared raw materials in it;
- take the end of the tube into the second vessel, which has 2 tubes (for removing gases and receiving liquid fuel);
- fill the third vessel with water as a condenser;
- on the lid of a vessel with two tubes, place the first end 1-2 cm;
- connect the condenser tube to the gas exhaust tube;
- place the second condenser tube under the first vessel and connect it to the gas burner;
- place the tube from the first vessel in a pipe of the largest diameter, because water will flow through it for cooling;
- light the main burner so that water begins to flow into the cooling circuit and the rubber turns into steam.
When passing through the pipe, the gas will cool, flow into the second vessel in the form of condensate, and flow through the outlet tube to the bottom of the condenser.
When the rubber in the first vessel runs out, turn off the water and burner.
Of course, you cannot get high-quality fuel from tires. But for refueling a chainsaw or heating a room, prepared gasoline is quite suitable.
Attention! The method cannot be used in closed rooms, apartments or private houses. In the process, smoke and fumes will rise into the air.
Making biodiesel at home
First of all, it is important to initially understand the difference between the same oil and biodiesel fuel itself. Vegetable oil (SVO), waste vegetable oil (WVO) and similar animal fats are naturally capable of powering a diesel engine, but they are not biodiesel fuels per se.
Why premium gas is a waste of money for most cars
In the first option, modifications to the engine itself cannot be done. At a minimum, a system of coarse and fine filtration of vegetable oil waste will be required. The option is not very good for the motor.
It is preferable to produce this biodiesel from SVO or WVO oils. The process is more complex and involves "breaking down" the chemical structure of fats or oils using methanol and lye. It is important to take the necessary precautions as both methanol and lye are toxic substances.
The process of making biodiesel from SVO, in its most basic terms.
-Heating the oil;
-Adding a certain amount of mixed ingredients of methanol and alkali, they will facilitate the chemical process known as transesterification;
-The result of this process will be that two products will ultimately be released, namely: biodiesel and glycerin, which will separate and settle to the bottom of this mixture;
-The final stage is drying of methyl esters of fatty acids. Since water itself leads to the development of microorganisms in biodiesel and promotes the formation of free fatty acids, which subsequently cause corrosion of metal parts.
Store for no more than 3 months.
Security measures
- Methanol is the strongest poison. If you accidentally swallow 30 ml, it can be fatal. When working with liquid, it is important to follow safety rules, wear a respirator, and avoid contact with eyes and mouth.
- The process of making ethanol at home is similar to moonshine brewing. Assumes using a torch. Experiments should be carried out away from flammable objects and materials.
- It is unacceptable to use open fire sources when producing fuel from oil.
Of course, producing gasoline at home is difficult. More likely, you will end up with fuel derivatives that will not run the vehicle. To obtain a pure homemade product you will have to work hard. It is important to have not only technical skills and the necessary equipment, but also patience and ingenuity. It is recommended to first read professional literature about the intricacies and nuances of this production. In addition, the business may become unprofitable and even illegal. To produce surrogate fuel in large volumes, finance will be required to purchase expensive equipment and raw materials. The costs will be no less than buying regular gasoline for your car at the nearest gas station.
see also
- Alternative automobile fuel
- Synthetic natural gas
- The methanol economy is a hypothetical future energy economy in which fossil fuels are replaced by methanol.
- Dry distillation
- GTL (Gas-to-liquids) is the process of converting natural gas into high-quality, sulfur-free motor fuels and other (heavier) hydrocarbon products.
- Hydrolysis production
- Biofuel
- Global Energy
- Solar oven is a simple device for using sunlight to cook food without using fuel or electricity.