In any heating network there are foreign particles circulating along with water through pipes and radiators. This is rust, salt sediment from the boiler (scale), all sorts of solid impurities. If the amount of debris is large, the heat exchangers begin to clog, and silt deposits form in the batteries and pipelines. They interfere with the flow of coolant and normal heat transfer. The solution to the problem is flushing the heating system of a private home, which you can do yourself.
Instructions for flushing the heating system
Pneumatic pulse cleaning scheme
There are 2 main methods for flushing the heating system, namely:
- using special hydropneumatic equipment;
- using chemical reagents.
Flushing using the hydropneumatic method
Hydropneumatic flushing of heating systems - instructionsHydropneumatic flushing of heating systems - instructions
This method is actively used by domestic housing offices and is quite effective. You just need to do everything in accordance with technology.
The principle is extremely simple: first, water is discharged from the system, then it is supplied back. A special pneumatic pump is used to “adjust” the water flow. As a result, under the influence of a fairly powerful pressure, scale and other deposits peel off, and when the water is drained, they are removed from the system.
To carry out this procedure yourself, you will need a pneumatic pump capable of pumping a pressure of more than 6 kg/cm2.
The sequence of actions is as follows.
Before starting work, you need to turn off all the taps. The end fittings are unscrewed using a wrench.
First step. We close the return valve.
Heating system line diagram
Second step. We connect the pneumatic pump to the valve installed after the valve.
Third step. We reset the return line.
Fourth step. Let the pneumatic pump build up pressure above 6 kg/cm2, and then open the valve to which it is connected.
Fifth step. We close off all the risers one by one. We do this so that no more than 10 risers are blocked at one time. Compliance with this rule will make the washing procedure as effective as possible.
Sixth step. We switch the system to reset in the opposite direction. To do this we do the following:
- close the discharge and close the valve connected to the pump, and turn off the device;
- close the open valve, and then open a similar one on the “return”;
- we reset the heating system. To do this, connect the pneumatic pump to the valve in the opposite direction, then open the valve and turn on the pump. The liquid will move in a different direction.
You can determine the required duration of rinsing by eye. Has clear clear liquid started coming out of the system? We can finish! Return the gates and valves to their original positions and turn off the pump.
Prepare a suitable container to collect dirty water. If you wish, you can connect a hose to the battery and ensure that the dirty coolant is discharged into the sewer.
Chemical wash
Chemical pipe flushing diagram
This method can be used only in two cases, namely:
- if it is necessary to clean a heating system with natural circulation, built using steel pipes. It is advisable to use chemical reagents in situations where, for any reason, there is no desire to flush the entire system. Most often, blockages are deposited in heat exchangers. The system can silt around the entire perimeter. In the second case, chemical washing will not be of much use;
- if it is necessary to restore the old heating system. Over decades of operation, pipes can become clogged and overgrown so much that the power of the pneumatic pump will not be enough for effective cleaning. It would, of course, be possible to use a more powerful pump, but no one can guarantee that the pipes will not burst under such pressure.
Wash reagent
The principle of flushing is simple: instead of coolant, a special solution containing acid and alkali is poured into the system. Then the mixture is circulated for 2-3 hours (if it is not the natural circulation line that is being cleaned, you will need to connect a pneumatic pump for this), after which it is drained and the pipes are filled with standard coolant.
Reagents for flushing and protecting heating systems
Never use such chemical mixtures to clean aluminum pipes. If the products remain intact after such washing, they will serve significantly less.
It is recommended to carry out mandatory flushing of the system of a private home at least once every 7 to 10 years.
Chemical flushing of industrial boilers - Overview.
Authors :
Mo D. Majnouni, Aramco Service Company, and Arif E. Jaffer, Baker Petrolite Corporation
Abstract
In order to ensure efficient heat exchange, the internal surfaces of boilers in direct contact with water and steam must be kept clean and free of deposits.
This article provides guidance on when and how it is appropriate to chemically flush boilers. Several methods can be used to determine the need for chemical boiler flushing.
The fire (frontal) surface of boiler pipes usually consists of magnetic iron ore (magnetite) and copper. The article emphasizes the cost effectiveness of various chemical cleaning agents for boilers contaminated with various types of deposits. Chemical boiler flushing must be carefully planned. The criteria that determine the success of chemical procedures and the effectiveness of reagents for dissolving certain deposits are determined on the basis of detailed analysis.
Dear potential Customer, if you have any questions about how to flush boilers, boilers or steam generators, immediately* call tel. or +7(495) 776-50-79.
There is no secretary on these phones; you will be answered by an experienced employee who will competently advise you on how to flush your boilers and whether they need flushing.
*Provided that you call on a working day before 18-00, or at least before 19-00.
Introduction
The appearance of deposits and scale in boilers is an inevitable, progressive process. Even with good water treatment and strict control of condensate using chemical additives, scale and deposits will appear.
Deposits cause the following main problems:
-Increasing the temperature of the pipe walls;
-Reduced heat transfer, leading to an increase in energy costs and loss of reliability.
The increase in pipe wall temperature occurs as a result of the low thermal conductivity of the deposits compared to the metal.
Reduced heat transfer can cause the design temperature of the pipe walls to be exceeded, which can ultimately lead to pipe failure due to creep failure. Heat transfer efficiency is defined as the ratio of boiler output to fuel consumption, once deposits begin to reduce heat transfer, more fuel will be required to produce the design temperature, i.e. There is a general loss of efficiency and loss of energy. Ultimately, removing deposits and scale from the boiler becomes imperative to prevent damage. One way to remove deposits and scale is to chemically flush the boiler. Chemical boiler flushing is a multi-stage process aimed at removing all existing types of deposits from the internal surface of boilers, as a result of which the hydraulic system should remain clean and decontaminated.
This article reviews the criteria for chemically cleaned industrial hot water boilers with operating pressures up to 900 psi. inch (63.3 kg/sq.cm), which are used to generate steam and are used in factories. The article talks about the main stages of chemical cleaning and the selection of specific chemical reagents that should be used at each specific stage, with special attention paid to the removal of iron and copper scale.
Chemical composition of deposits in boilers.
The main component of deposits in boilers is magnetite (Fe3O4), which is formed as a product of the reaction of metallic iron with high-temperature steam. Other crystalline materials, some of them are listed in Table. 1 may also form deposits. Copper is present due to corrosion of copper alloys in aluminum bronze feedwater condensers and preheaters, often due to oxygen infiltration into these systems. Copper is transported through the steam unit, where its particles settle on the internal surfaces of the boiler. Other components presented in Table. 1, settle on the internal surfaces of the boiler in the same way, and in addition they can occur from contamination of the feed water or the use of outdated water treatment products based on phosphoric acid salts. In addition to crystalline inorganic compounds, organic sediment may be present in sediments.
| Table 1: Composition of boiler deposits | ||
| Components | Formula | |
| Anhydrite | Anhydrite | CaSO4 |
| Aragonite | Aragonite | CaCO3 |
| Brucite | Mg(OH)2 | |
| Copper | Copper | Cu |
| Calcite | Calcite | CaCO3 |
| Hematite (red iron ore) | Hematite | Fe2O3 |
| Hydroxyapatite | Hydroxyapetite | Ca10(OH)2(PO4)6 |
| Magnetite (magnetic iron ore) | Magnetite | Fe3O4 |
| Quartz | Quartz | SiO2 |
| Trinardite | Thenardite | Na2SO4 |
| Volostanitis | Wollastonite | CaSiO3 |
When developing a chemical wash plan, it is necessary to take into account the various components present in the sediment in order to select the optimal chemical reagents.
The reagents recommended based on the analysis should effectively remove deposits and scale without damaging the metal underneath. Determining the need for chemical flushing of the boiler.
The need for chemical flushing is identified during routine inspections of the working parts of the boiler. The inspection should identify the problem areas of the boiler that are most affected by corrosion or contain the largest amount of deposits. Other factors influencing the decision to conduct inspections:
— loss of overall efficiency;
— the presence of areas of overheating - for example, evidenced by infrared studies;
— destruction of pipes during normal operation.
If data obtained during a routine inspection indicates that a chemical inspection may be necessary, collecting boiler tube cuttings is the most reliable method of performing a preparatory analysis. Pipe cuttings should be taken from problem areas. The length of the pipe cut should be at least 3 feet (about 1 meter) to ensure that the sampling tools (cutting wheel or cutting torch) do not contaminate the scale in the center of the cut with scale or filings. Then the deposits must be analyzed; there are several methods for determining their chemical composition.
The density of scale is determined by the gravimetric method after dissolution in inhibited hydrochloric acid. The weight loss when heated in the oven determines the percentage of hydrocarbons, which then determines the need for alkaline degreasing. The need for cleaning is determined based on sediment density analysis. Table 2 shows the sediment density scale according to the required actions.
| Table 2. Sediment Density and Adequate Action Scale | |
| Sediment density g/sq.ft (mg/sq.cm) | Recommended Actions |
| < 23 (25) | There is no need to do anything |
| 23-70 (25-75) | Chem. flushing for one year |
| 70-93 (75-100) | Chem. flushing within 3 months |
| >93 (100) | Chem. flushing before resuming operations |
Steps for Chemical Boiler Flushing and Treatment Selection .
Boiler cleaning usually consists of a combination of the following steps:
— Mechanical cleaning
— Washing with water
— Treatment with alkalis
— Cleaning with organic solvents
— Neutralization and passivation
Mechanical cleaning and water flushing can remove loose scale and other unconsolidated deposits from the interior surface of the boiler. Alkaline treatment removes oils and hydrocarbons that may prevent acidic washes from dissolving deposits. At the organic solvent cleaning stage, deposits are removed from the boiler using inhibited acid-based reagents. Once they are removed, the newly purified active metal becomes unprotected. A neutralization and passivation step is used to remove any remaining traces of iron oxide and coat the active metal with a well-passivated layer.
The composition of deposits, their quantity and distribution vary greatly from boiler to boiler and even within the same boiler at different periods of its operation. Therefore, it is necessary in each case to carry out a specific wash or series of washes in order to most effectively clean the boiler thoroughly and safely, according to the specified standards. This part of the article serves as a guide to choosing types of cleaning.
The main criteria that must be met are:
— Cleaning must be safe and compatible with the materials from which the equipment being washed is made.
— Sediments must demonstrate sufficient solubility during the selected type of cleaning. It is necessary to pay attention to the possibility of the formation of any insoluble substances during the reaction and to prevent their formation, which will allow achieving the specified degree of purification.
Satisfying these criteria, make your final choice, however, it is worth taking into account other criteria such as cost, waste disposal problems and time allocated for washing.
Chemical cleaning typically involves one or more of the following steps:
- hot alkaline degreasing,
- removal of copper,
— washing with acid-based reagents followed by neutralization and passivation
Laboratory studies will determine the sequence of chemical treatment steps.
Hydrodynamic flushing can be used to remove uncemented deposits. If hydrodynamic washing has been carried out, it should be followed by removal of corrosive deposits and passivation. Remote fiberscope surveys, videotaped and sediment density analysis of pipe cuttings, before and after chemical flushing, will provide visual confirmation of the effectiveness of chemical flushing. Hot alkali treatment stage.
If oil, grease, carbon or any organics are present, they must be removed through a chemical cleaning process. The choice of method depends on the degree of contamination. Hot caustic treatment is used only in cases where organic deposits would interfere with the effectiveness of chemical cleaning. If the solubility of the scale in the wash agent is greater than 70% with or without the addition of surfactants, a separate hot caustic treatment step is not required.
Degreasing with soda ash (Na2CO3) is a mild treatment used in cases where the contamination initially consists of light oil or grease; with less than 5% content of organic contaminants. Table 3 shows control parameters for alkaline degreasing with soda ash.
| Table 3. Control parameters for alkaline degreasing with soda ash. | ||
| Chemicals | Concentration | |
| Sodium carbonate | Sodium carbonate | From 0.5 to 1.0% by weight |
| Sodium metasilicate | Sodium metasilicate | From 0.5 to 1.0% by weight |
| Trisodium phosphate | Trisodium phosphate | From 0.5 to 1.0% by weight |
| Surface-active substance | Surfactant | From 0.1 to 0.2% of volume |
| Defoamer (if required) | Antifoam (if required) | From 0.05 to 0.1% of volume |
| Temperature limit | Temperature Limit | 155 °C |
| Circulation | Normal working | |
| Processing time | 18 to 24 hours | |
| Corrosion rate | Corrosion Rates | <2 mpy |
Caustic soda (NaOH) degreasing is commonly used on all new boilers; if secondary scale is present or if contamination is from 5 to 30%. Table 4 shows control parameters for caustic soda degreasing.
| Table 4. control parameters for degreasing with caustic soda. | ||
| Chemicals | Concentration | |
| Sodium hydrate (caustic soda) | Sodium hydroxide | 1.0 to 2.0% by weight |
| Trisodium phosphate | Trisodium phosphate | From 0.5 to 1.0% by weight |
| Surface-active substance | Surfactant | From 0.1 to 0.3% of volume |
| Defoamer (if required) | Antifoam (if required) | From 0.05 to 0.1% of volume |
| Temperature limit | Temperature Limit | 155 °C |
| Circulation | Normal working | |
| Processing time | 18 to 24 hours | |
| Corrosion rate | Corrosion Rates | <2 mpy |
Potassium permanganate (KMnO4) is used where the amount of organic contaminants is significant (>30) and coked. This treatment should only be used where the type and quantity of contaminants cannot be removed otherwise, as the cost, disposal problems and complexity of subsequent chemical washing are significantly higher than alternative options. Control parameters for alkaline degreasing with permanganate are shown in Table 5
| Table 5. Control parameters for alkaline degreasing with permanganate. | ||
| Chemicals | Concentration | |
| Sodium hydrate (caustic soda) | Sodium hydroxide | 1.0 to 2.0% by weight |
| Potassium permanganate | Potassium permanganate | 1.0 to 3.0% by weight |
| Temperature limit | Temperature Limit | 100 °C |
| Circulation | From1200 l/min to 4500 l/min | |
| Processing time | From 6 to 12 hours | |
| Corrosion rate | Corrosion Rates | <2 mpy |
Where sediments contain large amounts of calcium sulfate (i.e. 10%), sulfate conversion treatment may be necessary and economically feasible. It will help increase scale solubility during subsequent treatment with acidic washes such as inhibited hydrochloric acid. Control parameters for sulfate conversion treatment are shown in Table 6.
| Table 6. Sulfate Conversion Processing Control Parameters | ||
| Chemicals | Concentration | |
| Sodium carbonate | Sodium carbonate | From 1.0 to 5.0% by weight |
| Surface-active substance | Surfactant | From 0.1 to 0.2% of volume |
| Temperature limit | Temperature Limit | 95°C |
| Circulation | From1200 l/min to 4500 l/min | |
| Processing time | 12 to 24 hours | |
| Corrosion rate | Corrosion Rates | <2 mpy |
If the copper concentration in the deposits is greater than 10%, separate treatment is necessary to dissolve as much copper as possible before cleaning with acid washes. Estimate the copper that needs to be removed based on sediment analysis and use one of the following alkali treatments to reduce copper levels to less than 10%. Other alkaline treatments with ammonium carbonate and sodium nitrate are also suitable for removing copper at concentrations greater than 10%. The remaining copper in the boiler will be removed during the acid washing process. Table 7 shows control parameters for copper removal with ammonium bicarbonate, air or oxygen.
| Table 7. Control parameters for copper removal with ammonium bicarbonate, air or oxygen. | ||
| Chemicals | Concentration | |
| Ammonium bicarbonate | Ammonium bicarbonate | 1.6 l/kg copper removed |
| Ammonia-water solution (ammonia) | Aqua ammonia | 2.4 l/kg copper removed pH = 9.5 |
| Air or oxygen | From 1.3 to 1.5 cubic meters per minute | |
| Temperature | 50-60°C | |
| Time of processing | From 2 to 4 hours | |
| Corrosion rate | <2 mpy | |
Stage of chemical cleaning with acid reagents.
Hydrochloric acid –
Since inhibited hydrochloric acid is produced with good dissolving ability towards a wide variety of scales, inhibited hydrochloric acid is the most widely used scale dissolving reagent. This is an economical and easy-to-manage type of chemical wash. When the parameters are followed and when inhibitors are added correctly, this method shows good corrosion performance. The process is quite flexible and can be tailored to remove copper compounds by adding thiourea (H2NCSNH2), to enhance silica removal by adding ammonium bifluoride, or to enhance organic removal by adding a surfactant. This type of washing is not compatible with stainless steel. When the concentration of copper in deposits is more than 10%, separate removal of copper is necessary before using hydrochloric acid. Table 8. Shows control parameters for hydrochloric acid washing.
| Table 8. Control parameters when washing with hydrochloric acid. | ||
| Chemicals | Concentration | |
| Hydrochloric acid | Hydrochloric acid | 3.5 -7.5% by weight |
| Inhibitor | Inhibitor | 0.2 - 0.3% by weight or as recommended by the manufacturer |
| Surfactant | Surfactant | 0.0 to 0.2% by volume |
| Ammonium hydrodifluoride | Ammonium bifluoride | 0.0 to 1.0% of weight |
| Thiourea (H2NCSNH2) | Thiourea | 0.0 to 1.5% of weight (up to 5 kg/kg copper removed) |
| Oxalic acid | Oxalic acid | 1.0% by weight |
| Temperature | 70°C to 82°C | |
| Circulation speed | 1200 l/min to 4500 l/min | |
| Time of processing | From 8 to 18 hours | |
| Corrosion rate | Corrosion Rates | <600mpy |
| Total dissolved iron | Total dissolved Iron | 10,000 mg/l maximum |
Citric Acid -
Citric acid is compatible with alloy steel and requires low levels of chloride solvents and is easy to use, safer and has better anti-corrosion properties than hydrochloric acid. It is less aggressive in dissolving iron oxide deposits and also requires higher temperatures and longer contact times. It has a very limited effect on calcium salts in sediments. And it is usually a more expensive procedure compared to hydrochloric acid cleaning.
Common reasons for choosing citric acid cleaning:
— Availability of austenitic materials of construction.
— The need for the most efficient removal of copper from heavily copper-contaminated deposits
Reduces the overall time of cleaning procedures because there is no need to drain the water, rinse with water and refill the boiler, since the removal of iron, copper, neutralization and passivation can be done with one solution.
Control parameters for citric acid washing are given in Table 9.
| Table 9. Control parameters for cleaning with citric acid. | |
| Chemicals | Concentration |
| Iron removal phase | |
| Lemon acid | 2.5-5.0% by weight |
| Inhibitor | 0.2 – 0.3% by volume or recommended by the manufacturer |
| Ammonium | Add enough to pH 3.5 to 4.0 |
| Copper removal and passivation phase | |
| Ammonium | Add enough to pH 9.2 |
| Ammonium bicarbonate | 1.0% by weight |
| Sodium nitrite | 0.5% by weight |
| Temperature limit: — Iron removal phase — Copper removal and passivation phase | 79°C - 93°C 45°C - 50°C |
| Circulation | 1200 l/min to 4500 l/min |
| lead time | |
| Iron removal phase | 4-8 hours |
| Copper removal phase | 4-8 hours |
| Total dissolved iron | 10,000 mg/l maximum |
| Circulation speed | 1200 l/min to 4500 l/min |
| Corrosion rate | <660mpy |
EDTA –
Cleaning with
ethylenediaminetetraacetic acid
is typically more expensive than citric or hydrochloric acid. In addition, higher temperatures are required to obtain a sufficient degree of purification. Corrosion rates are low, below properly controlled conditions; removal of iron oxides; Copper removal, neutralization and passivation can be achieved sequentially in one solution. EDTA circulates during normal boiler operation and air purge. The need to provide temporary circulation pumps, connection work and tapping into pipes is thus eliminated.
Table 10 shows the control parameters for EDTA purification.
| Table 10. Control parameters for EDTA purification. | |
| Chemicals | Concentration |
| Iron removal phase | |
| EDTA | 3-10.0% by weight |
| Inhibitor | 0.2 – 0.3% by volume or recommended by the manufacturer |
| Ammonium | Add enough to pH 9.2 |
| Copper removal and passivation phase | |
| Sodium nitrite | 0.5% by weight |
| Temperature limit: — Iron removal phase — Copper removal and passivation phase | 121°C - 149°C 60°C - 71°C |
| Circulation | Normal circulation |
| lead time | 12-18 hours |
| Corrosion rate | <200mpy |
Sulfuric Acid –
Sulfuric acid is a very effective solvent for iron oxides, iron sulfides and is usually less expensive than hydrochloric acid. It can be used to clean stainless steel. However, it is much more dangerous to use. Sulfuric acid in this concentrated form is very aggressive and contact with skin or eyes is very dangerous. Sulfuric acid flushing is not recommended if deposits contain large amounts of calcium due to the formation of insoluble calcium sulfates. Table 11 shows control parameters for sulfuric acid treatment.
| Table 11. Control parameters for treatment with sulfuric acid. | |
| Chemicals | Concentration |
| Sulfuric acid | 4.0 – 8.0% by weight |
| Inhibitor | 0.2 – 0.3% by volume or as recommended by the manufacturer |
| Surfactant | 0.0 – 0.2% of volume |
| Temperature limit | 60°C - 82°C |
| Circulation speed | 1200 l/min to 4500 l/min |
| lead time | From 4 to 12 hours |
| Corrosion rate | <600mpy |
| Total dissolved iron | 10,000 mg/l maximum |
Sulfamic Acid –
Sulfamic acid has the advantage that, being a crystalline solid, it is easy to store, apply and mix. It is often sold with an inhibitor and a color indicator to determine the strength of the sulfuric acid solution. This acid is compatible with stainless steel and is a moderately aggressive solvent for iron oxides and calcium carbonates. Due to its relatively high cost, it is usually used for flushing small-volume equipment. Control parameters for sulfamic acid are given in Table 12.
| Table 12. Control parameters for sulfamic acid. | |
| Chemicals | Concentration |
| Sulfamic acid | 5.0 – 10.0% by weight |
| Inhibitor | 0.1 – 0.2% by volume or as recommended by the manufacturer |
| Surfactant | 0.0 – 0.2% of volume |
| Temperature limit | 55°C - 65°C |
| Circulation speed | 1200 l/min to 4500 l/min |
| lead time | From 4 to 12 hours |
| Corrosion rate | <600mpy |
| Total dissolved iron | 10,000 mg/l maximum |
Hydrodynamic cleaning (HDC)
Very effective cleaning for removing uncemented deposits. The use of HDO is recommended after chemical washing with acid-based reagents and neutralization. After using GDO, it is necessary to remove rust deposits and passivate the boiler before starting it up. Table 13 shows the control parameters of GDO.
| Table 13. GDO control parameters. | |
| Equipment | Specification |
| Pump characteristics | 750 kW, 68.94 MPa (10,000 psi) 1500 kW, 137.88 MPa (20,000 psi) |
| Water volume | From 30 l/min to 50 l/min |
| Hole diameter | 0.8 to 2.4 mm |
| Maximum distance between the nozzle tip and the boiler surface to be cleaned | 25 mm |
| Flexible hose diameter | 19 mm minimum |
| Rinse water | Cold steam condensate |
| Supplements | Concentration |
| Polymer | 0.3 of volume |
| Surfactant | 0.1-0.2 of volume |
Neutralization and passivation.
After flushing the boiler with acid-based reagents, it is necessary to carry out neutralization. To perform neutralization only, 0.5% sodium carbonate is usually used; if the pH is 7 or more, then passivation is required, neutralization is achieved during its implementation. The choice of passivation process is influenced by the choice of acid reagent used for chemical washing. After the use of citric acid or EDTA reagents, a long-term neutralization and passivation effect usually occurs with acceptably adjusted pH and the addition of an oxidizing agent. The use of reagents based on citric acid, ammonium and nitrites or reagents with carbon dioxide salt and nitrites must end with neutralization and passivation. As long as the temperature does not rise above ambient temperature, a nitrite phosphate treatment will provide some protection to metal surfaces. If the surfaces have been rusted, the rust is first removed with citric acid, ammonia and sodium nitrite are added later in order to achieve a higher degree of passivation.
The corresponding control parameters are presented in Tables 14, 15, 16.
| Table 14. Control parameters for neutralization with carbon dioxide salt. | |
| Chemicals | Concentration |
| Sodium carbonate | 0.5-1.5% by weight |
| Sodium nitrite | 0.5% by weight |
| Temperature limit | 88°C to 93°C |
| Circulation speed | 1200 l/min to 4500 l/min |
| Time of processing | From 8 to 12 hours |
| Corrosion rate | <2 mpy |
| Table 15. Control parameters for neutralization with phosphates and nitrites. | |
| Chemicals | Concentration |
| Sodium nitrite | 0.5 by weight |
| Monosodium phosphate | 0.25% by weight |
| Disodium phosphate | 0.25% by weight |
| Sodium hydroxide | Bring pH to 7 |
| Temperature limit | 50°C to 65°C |
| Circulation speed | 1200 l/min to 4500 l/min |
| Time of processing | From 8 to 12 hours |
| Corrosion rate | <2 mpy |
| Table 16. Control parameters for neutralization with citric acid, ammonia and sodium nitrate. | |
| Chemicals | Concentration |
| Lemon acid | 2.5 by weight |
| Ammonia | Reduce to 4 during rust removal process and bring pH to 9.5 during passivation process |
| Sodium nitrite | 0.5% by weight |
| Inhibitor | 0.2 – 0.3% by volume or according to manufacturer’s recommendations |
| Temperature limit | Rust removal from 65°C to 90°C |
| Passivation stage | from 45°C to 50°C |
| Circulation speed | 1200 l/min to 4500 l/min |
| Time of processing | From 8 to 18 hours |
| Corrosion rate | <600 mpy in rust removal process and lead <2 mpy in passivation process |
Evaluation of chemical cleaning.
Inspecting the boiler after chemical flushing is a key point in determining the success of the procedure. The effectiveness of flushing is determined visually or using a borescope (a device for inspecting the surface of deep holes). There should be no visible traces of water or loose or coked deposits inside boiler drums and pipes. Remove corrosion test pieces and polarization samples, visually inspect them, determine their weight loss, and calculate the metal thickness loss (typically <25 microns) due to chemical rinsing.
Density of deposits after cleaning:
-cut out the pipe after flushing and determine the density of deposits. Use Table 17 to evaluate the effectiveness of chemical flushing.
| Table 17. Evaluation of the effectiveness of chemical washing. | |
| Presence of sediments, g/ft; (mg/sq.cm) | Performance evaluation |
| 0.93 (1.0) or lower | The best |
| Between 0.93 – 1.86; (12) | The best |
| Between 1.86 – 2.79; (2 – 3) | good |
| Between 2.79 – 4.65; (3 – 5) | Acceptable |
| > 4.65; (5) | Unacceptable |
| Passivation stage | Removing rust at 65°C to 90°C |
conclusions
For efficient operation of equipment, control of corrosion, reliability and prevention of accidents, it is necessary to flush boilers periodically. Purification is achieved by performing a combination of several stages. In some cases, it is necessary to use all stages of flushing; the degree of contamination of the boiler can vary greatly from one to another. The exact procedures that should be used depend on the density of the sediment and the result of its analysis, swelling or broken pipes, water treatment analysis, inspection and history of the equipment being cleaned.
The recommendations formulated in the article do not replace the recommendations of boiler manufacturers regarding their cleaning, especially if the warranty period is valid.
About the Authors
Moe D. Majnauni is a water treatment engineer at Aramco Service Company in Houston. Arif E Jaffer is a senior consultant at Baker Petrolite Corporation in Texas.
Translation of an article published in The ANALIST-the voice of the water treatment industry;
Autumn 2004, Number 4.
Other articles
Flushing heat exchangers
Boiler cleaning cost
Cleaning heat exchangers
Cleaning and washing of floor-standing boilers
Removal of scale and soot is carried out without dismantling the heat exchanger. A flushing pump (booster) is used. An aqueous solution of citric acid is poured into its container. For 2 liters of warm water, 200 grams of powdered citric acid is required.
Before cleaning a floor-standing gas boiler, turn off the gas and water supply taps, drain the water from the heating and hot water circuits. Next you need to get to the heat exchanger. Remove the door, disconnect the wires connected to the piezoelectric element, remove the thermocouple and nozzle, dismantle the ignition system and burner.
Unscrew the nuts securing the top cover and remove it. You have gained access to the heat exchanger and can clean it of soot with a brush and brush.
Connect the leads of the booster to the heat exchanger pipes, which will pump a citric acid solution into the pipe under pressure. Circulating along the circuit for 4-6 hours, it will dissolve the scale. The rinsing time depends on the level of contamination.
To keep the process under control, use a pH meter. This device will show the change in acid concentration in the solution, which occurs as a result of the ongoing chemical reaction of dissolving carbonate deposits.
If the acid has neutralized and the meter displays a pH value of 1, you may have to repeat the process all over again. A stable pH level between 2 and 4 indicates descaling.
Finally, the pipes are washed with a baking soda solution to neutralize residual traces of the reagent. Next, it remains to install the parts in their places, check the unit for leaks by washing and visually inspecting the detachable connections, or using crimping. If there are no gas or water leaks, the boiler operates as usual.
What are the restrictions on hydrodynamic cleaning?
Hydraulic cleaning of boilers is applicable only to drum steam boilers E, DE, KE or DKVR.
This is due to the fact that the above boilers have access to screen and convection tubes. In boilers E, DE, KE or DKVR, the specialist performing hydrodynamic cleaning of the boiler is located inside the drum and performs work on even the most inaccessible tubes.
In this regard, there are restrictions for water heating boilers PTVM, KVGM, ZIO for cleaning using a hydrodynamic method, since the collectors of these boilers do not have hatches for comfortable access for a flushing specialist.
For the same reason, some fire-tube boilers cannot be washed completely, but only from the top or from the end using hydrodynamic cleaning, because it is in these places that they have access to the pipes. These include the following boilers: Booster, Ferolli, Babcock Wanson, De Dietrich, Buderus, ICI, IVAR, BOSH UL-S, Unical Bahr, Viessman.
Most often, high-pressure hydrodynamic cleaning is carried out in combination with chemical treatment of the boiler, when something that could not be removed by chemical washing is removed.
Peculiarities
Flushing the heating system cannot be done “blindly”, because you need to know what you will encounter. Typically, batteries do not heat up well when the fluid circulation in them is weak due to debris clogging the internal walls. It is found in the water itself in the form of suspensions of alkali salts, heavy metals and rust. Utilities are required to flush the system annually to prevent a decrease in water flow. But often this task has to be performed by the residents themselves, inviting specialists to clean the radiators from the dirt compacted inside.
This problem is especially typical for cast iron batteries, where sometimes there is a channel for water circulation no more than 1 cm in diameter due to infrequent washing. The cause of clogging of steel radiators is stagnation of rust, due to which they are subject to not only clogging, but also destruction. Not every type of flushing is suitable for such batteries, since after some methods leaks may appear in them. Plastic radiators also become clogged, but cleaning them differs from cleaning their metal counterparts.
This process cannot be called universal, because it is selected specifically for each case. An example of a bad choice is the flushing of the heating system by housing and communal services workers, when they apply powerful pressure by abruptly opening the riser valve located in the basement of an apartment building. This leads to knockout of locking elements and leaks at the junctions of spans. But not every family owner can disassemble the battery into separate bends for the purpose of mechanical cleaning in order to solve the problem of a clogged heating system.
When flushing radiators in an apartment on the lower floors, one cannot always expect the efficiency of the coolant, since for high-quality cleaning it is necessary that the neighbors of all floors of the same branch do the flushing. Otherwise, after cleaning one apartment and washing the batteries by utility workers with high pressure, some of the garbage may fall behind the walls, and mud masses will fall into the newly cleaned batteries. However, it is unlikely that all neighbors will agree when it comes to flushing, but the insufficient internal cross-section creates high hydraulic resistance.
Every mm in thickness of deposits increases fuel consumption by 20-25%. In central heating systems, the water must be pre-treated to reduce clogging. But this is not always done, although residents pay decent amounts every month for house maintenance. In addition, radiators last for decades without replacement. According to the established regulations, flushing of centralized and autonomous systems must be carried out annually. This period is critical. If the pipes are not flushed before the start of the heating season, the pipeline becomes clogged, causing the heating equipment to break down.
If you ignore the blockage, the consequences can be disastrous:
- the heating system may freeze;
- you will have to buy new batteries;
- You will need an electric heater, which will increase the price of electricity.
How to properly flush the heat exchanger of a double-circuit boiler?
Mikhail, Ekaterinburg asks a question:
Good afternoon 3 years ago we installed a double-circuit boiler. This is a very convenient device, since the apartment is now warm and there is always hot water. At the time of purchase, I was told in the store that it is necessary to clean the boiler once a year if the tap water is very hard. To carry out cleaning, you need to call specialists, but this costs a lot of money. In fact, that’s why we didn’t clean the installation for so long. Tell me, at what intervals should cleaning be done? And how to rinse the heat exchanger of a double-circuit boiler from lime deposits? Thank you so much in advance for your help!
Hello! You should not worry too much, since modern models of double-circuit boilers do not deteriorate at all if you clean them once every 3 years (even if the water is very hard). The main thing #8211 is not to start the process until 5 years of use. The fact that the store advised you to clean it once a year is only partially correct. But this makes no sense, since you will only waste money again. The ideal option #8211 is to carry out such work once every 3 years, subject to constant use of the boiler.
It is necessary to understand how to rinse the heat exchanger under a double-circuit boiler correctly. There are several cleaning methods. The most effective is considered to be standard washing using a special liquid that is capable of peeling off limescale deposits from the internal walls of a given installation.
Before you get to work, you need to turn everything off. To begin, turn on the tap at the bottom of the boiler, which ensures the flow of cold water into it. Next you need to reduce the pressure to zero. If there is no such relief valve, skip this step. Now be sure to disconnect the boiler from gas and electricity. This must be done to ensure your safety. If possible, immediately drain the water from the boiler.
Now you need to remove the casing from the boiler. To do this, you need to unscrew the four nuts at the bottom. Please note that water may flow from below. Therefore, it is recommended to do this over a large basin or even take the boiler outside, first removing it from the hinges on the wall. Unscrew the heat exchanger slowly to avoid damaging the parts.
Next, the heat exchangers are washed. This can be done with ordinary running water. You will see for yourself when the water becomes cleaner after washing. Absolute clarity will indicate that the majority of the flushing was successful. Now you can rinse everything with boiled cold water.
Now it's time to use the cleaner. A special liquid for flushing circuits is purchased in advance, which eliminates deposits on parts. This liquid is poured into two basins, then the contours are placed in these basins so that their entire surface is covered with the product. Leave it all for 15-20 minutes. But before that, read the instructions carefully. After all, some products may require more or, conversely, less time for settling.
After this, the circuits are pulled out of the containers. Now, instead of liquid, you need to pour boiling water with washing powder diluted in it into the basins. The amount of powder should be sufficient. Place the heat exchangers in this solution for 3-5 minutes.
Now all you have to do is rinse the contours several times so that there are no traces of concentrate and powder left.
Flushing the double-circuit boiler can be considered complete. All you have to do now is install the heat exchanger in place. To do this, you first need to install new rubber gaskets, even if the old ones are still quite suitable.
Then everything needs to be returned to its place, trying to tighten the nuts as tightly as possible. A good way to do this is to use a wrench. Once everything is assembled, hang the cauldron back on the hooks. Then you can connect the installation to gas, electricity and water. You are convinced that figuring everything out is not so difficult. And if difficulties arise during assembly or disassembly of the circuits, you need to refer to the instructions. There is a diagram where everything is shown in detail, so it is almost impossible to get confused.
Chemical descaling of boilers
The chemical cleaning method, which involves preliminary determination of the composition of the scale and its nature, will help to clean the boiler quickly and effectively. This method is considered the simplest, fastest and most effective. Before cleaning, you need to take a scale sample from different places, and then start determining the average sample.
Carbon dioxide promotes the rapid dissolution of carbonate and phosphate deposits. Hydrochloric acid interacts with carbon scale, forming chloride compounds of calcium, magnesium and carbon dioxide, which easily dissolve. Cleaning scale from phosphate and silicate scales is more difficult, but efficiency can be increased by adding fluorine acid to the cleaner.
Types of acids for cleansing:
- Solyanaya;
- Sulfuric;
- Sulfamine;
- Sorrel;
- Lemon.
When choosing acids, it is important to pay attention to their availability, cost, effectiveness and environmental friendliness. You can clean the boiler chemically with your own hands, but you need to be extremely careful. The most popular in the West are reagents that belong to the class of chemical acids
Chemical cleaning is the most reliable and effective type of descaling if all reagents are selected correctly
The most popular reagents in the West are those that belong to the class of chemical acids. Chemical cleaning is the most reliable and effective type of descaling if all reagents are selected correctly.
Why does a gas boiler become clogged with soot?
The reasons for the formation of soot in boilers when burning natural gas may be different, but all of them, one way or another, are related to the property of the burned fuel, under certain conditions, decomposing into carbon and hydrogen. Modern heating units require precise adjustment of the burner device, taking into account the intensity of air supply and exhaust of combustion products.
The catalysts for soot formation are:
- Incorrect ratio of gas and air proportions during combustion.
- Poor quality fuel.
To reduce the percentage of soot in the flue gases, you will need to tune the boiler, check and, if necessary, replace the jets, and clean the nozzles from carbon deposits.
The formation of soot due to incomplete combustion of gas can be easily prevented if a specialist is invited to install and adjust the boiler. Every year, during seasonal maintenance, the operating parameters of the burner and the composition of the CO emitted are checked.
How to clean a gas boiler from soot deposits
Clean a gas boiler from soot at home? much easier than removing scale from the internal cavity of the heat exchanger. Carbon deposits are removed using the abrasive method. As a chemical reagent? use regular soda. Cleaning is done with a hard kitchen sponge.
You can dissolve soot with regular boiling water. If the carbon deposit thickness is more than 2 mm, special cleaning agents are used. First, the surface is treated with a metal brush. Through the furrows, the chemical solvent better penetrates the surface and removes soot.
How to clean nozzles on a gas boiler
There are several ways to clean carbon deposits on gas injectors:
- Mechanical method - this is how gas workers clean injectors during regular seasonal maintenance. The ash is removed with a special tool that resembles a curved hook.
- Using chemicals – heavy stains are removed with reagents. For cleaning, the burner device will need to be removed.
- The ultrasonic method is a relatively new cleaning method that allows you to get rid of carbon deposits with minimal consequences for the burner and the nozzles themselves. It is carried out on specialized equipment.
Which gas boiler heat exchanger is less susceptible to scale?
Scale appears on heat exchangers of any type. The rate and intensity of deposit formation depends on the material of the water circuit:
- Cast iron heat exchangers have the best resistance to the appearance of salt and mineral deposits. The service life of boilers is about 35 years. The circuit is made of cast iron, resistant to any aggressive substances.
- The heat exchanger is made of stainless steel and is resistant to corrosion, but after several years of operation, deposits of metal salts begin to appear on the walls of the circuit cavity.
- For copper heat exchangers, the first place in terms of the intensity of deposits is prepared. The process of scale formation is accelerated if aluminum fittings, adapters or radiators are used in the heating or hot water system.
Cost of flushing and cleaning a gas boiler
You can clean the DHW and heating heat exchanger yourself, but at the same time, there is a possibility of damaging the heating water circuit. Further repairs of boiler equipment will cost approximately half the cost of a new unit.
Independent cleaning work will cost the following amount:
- Chemical washing – 300-600 rub.
- Washing equipment (booster), starting from 15 thousand rubles.
- Brushes and sponges for cleaning – 50-100 rubles.
The effectiveness of chemical cleaning performed at home without specialized equipment is low. At best, it will be possible to reduce the scale layer. The risk of breaking something, especially when cleaning a plate heat exchanger for hot water, is very high. For this reason, independent maintenance is impractical and not economically profitable.
The best solution would be to seek help from a specialized service center. The boiler cleaning frequency is:
- Once every 2 years, in urban conditions.
- Every year, when installed in rural areas.
For this reason, companies providing technical support often draw up an annual service agreement, which includes not only flushing the circuit, but also making the necessary adjustments, etc. The cost of the contract, depending on the region, boiler and heat exchanger model, varies, starting from 8 thousand rubles.
Single-circuit or double-circuit boiler - is there a difference?
Varieties of boilers do not in any way affect the time period after which it is necessary to flush the heat exchanger. It is much more important which liquid () circulates in the heating system and which is supplied for hot water supply.
When using process water that has undergone standard purification, the boiler can be flushed no more than once every four years. This removes a layer of scale (which still forms) and deposits that have a more complex structure. If you do not filter water before pouring it into the system, but use ordinary water from a centralized water supply, then flushing should occur more often, at least once every two years. This is due to the high content of chlorine in the liquid, which, upon contact with the heating element, settles in the form of scale.
Some users prefer to use antifreeze as a coolant. This liquid is of higher quality: it does not freeze even at low temperatures, releases heat more slowly, but heats up quickly. Unfortunately, antifreeze is poisonous
, and breaking down into components, leads to damage to metal structures. The heat exchanger through which antifreeze circulates should be cleaned at least once every 1.5-2 years.
Therefore, both single-circuit and double-circuit boilers require timely cleaning of the heat exchanger, the intervals between which are the same in all systems.
In order to clean the bithermal heat exchanger less often, it is necessary to take care not only of the quality of the coolant in the heating circuit, but also of the quality of the water in the DHW system. The water should be pre-purified and filtered. You should also take into account the fact that the process of scale deposition begins at a temperature of 70°C, its rate increases by 2 times with every 10°C increase in temperature. In this case, the process progresses, since the growing layer of calcium impairs heat transfer and the temperature of the heat exchange wall increases.
Causes of pollution
What is scale? Solid calcium-based salt deposits that do not dissolve in water are called scale.
The main reason for the formation of such deposits is the use of unsuitable water, characterized by increased hardness.
This phenomenon is common and predictable - as a result of exposure to high temperature, a chemical reaction occurs and water, circulating through the heating system, leaves behind such traces. Not only pipes, but also the internal surfaces of the boiler are susceptible to the deposition of scale of varying chemical composition and structure.
This is mainly caused by the following factors:
- changing composition of the heating coolant and subsequent formation of solids;
- deposition on the internal parts of the boiler of various suspensions dissolved in water;
- metal corrosion of elements and the formation of dense corrosive deposits;
In addition to the above factors, the formation of deposits is provoked by the following reasons:
- quality of water supplied to the boiler;
- operating temperature and load on the thermal installation;
- boiler designs;
- operating mode.
What should be done in this case? The best way is to purify and prepare the water, but this procedure is very expensive and not simple. But there is a simpler and more convenient solution, which is inevitable, since scale cannot be avoided in the production process.
This solution is to chemically flush steam boilers from various deposits.
Chemical flushing is considered the most effective and convenient way to clean a steam boiler.
Flushing the boiler from scale: consequences of ignoring
Modern mains use ordinary hard water, which quickly leads to the inside of the equipment being covered with scale. Boilers must be cleaned on a regular basis. If cleaning is not done on time. The consequences can be the most unpredictable, but definitely unpleasant.
The design of the gas boiler is such that the coolant coming from the return line cools the cavities of the heating elements located inside. The coolant cannot effectively cool the elements if they are covered with a thick layer of scale. If the boiler constantly overheats, it will soon stop working altogether.
What will happen if you ignore flushing?
- Scale consists of mineral deposits that do not promote thermal conductivity. Scale causes the water to heat up slowly, which requires significantly more electricity. A thick layer of scale leads to increased gas consumption, which increases the price of using the boiler.
- Scale can lead to boiler failure due to difficult coolant passage. This increases the load on the circulation pump, which leads to its rapid breakdown.
Before flushing the boiler, it is important to pay attention to what kind of liquid flows through the line. The need for frequent flushing will be due to very hard and contaminated water. In order to reduce the frequency of cleaning, it is necessary to use antifreeze - it is important that it is not expired
In order to reduce the frequency of cleaning, it is necessary to use antifreeze - it is important that it is not expired
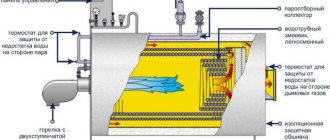
About the dangers of scale in a heat exchanger
Heating of tap water for a hot water supply system (DHW) in a double-circuit gas boiler or in a gas water heater is carried out in a flow-through heat exchanger.
It is known that when heated above 54 oC, crystallization of salts of chemical elements dissolved in water, mainly calcium and magnesium, occurs. Solid salt crystals settle on the heating surfaces of the heat exchanger and form a strong crust on them.
In addition to hardness salts, scale deposits include other solid particles found in water. For example, rust particles, oxides of other metals, sand, silt, etc.
The amount of salt in water determines the degree of its hardness. There are hard water, which contains a lot of salt, and soft water, with a small amount of salt.
If the source of tap water is a river or other natural body of water, then the hardness of such water is usually low. You are lucky, the water in your home is soft.
Tap water from a well usually contains more hardness salts. And the deeper the well, the more salt there is in the water.
A hard crust of hardness salts, rust, sand, silt on the heating surface of the heat exchanger prevents the transfer of heat through its metal walls. In addition, deposits reduce the clearance of the heat exchanger channels. As a result, the heating temperature and hot water pressure gradually decrease, and the walls of the heat exchanger overheat, which reduces its service life.
The internal structure of a double-circuit gas boiler using the example of Protherm Gepard 23 MTV and Panther 25.30 KTV (Panther). The secondary DHW heat exchanger is located in the lower compartment.
Double-circuit gas boilers most often have two heat exchangers. One is the primary one, in which water is heated with gas for heating. The other is the secondary DHW heat exchanger, in which heating water from the primary heat exchanger heats the DHW water from the mains.
There are also double-circuit boilers in which both water for heating and water for domestic hot water are heated with gas in one combined bithermic heat exchanger. A bithermic heat exchanger accumulates scale faster, and it is more difficult to clean it from scale.
The geyser has one DHW heat exchanger, in which tap water is immediately heated by gas.
Regular descaling is necessary only for the DHW heat exchanger, in which there is a constant accumulation of deposits of hardness salts.
In the channels of heat exchangers with heating water, scale accumulation occurs only when fresh water is replaced or added to the system. This happens quite rarely and in small volumes.
If there is a filter at the entrance to the heating water boiler, then other dirt from the heating system does not enter the boiler and there may be no need to clean the boiler coolant channels during its entire service life. It is not necessary to descale the primary heat exchanger at the same intervals as the DHW heat exchanger. However, “servicemen”, without proper reason, often insist on descaling the primary heat exchanger, at the same time, just in case. Naturally, they charge for this.
Secondary plate heat exchanger for hot water supply of a double-circuit gas boiler. Two holes serve to circulate heating water through the heat exchanger. Through the other two, cold water enters and heated hot water exits. Regular descaling of the inside is required.
Bithermic heat exchanger of a double-circuit gas boiler. On the right are pipes for heating water. On the left are the DHW water connections. Regular cleaning of scale inside and soot outside is required.
Heat exchanger for DHW gas water heater. Regular cleaning of scale inside and soot outside is required.
An effective way to descale
To carry out the highest quality cleaning of equipment, you can use a modern device - a booster. It will effectively solve the problem of how to flush a gas boiler from limescale.
What does the booster include:
- circulation pump;
- container for loading reagents;
- electric heating part.
The booster functions as follows:
- the reagent is heated in a special container (in order to increase its cleaning properties);
- the circulation pump pumps the heated reagent into the coil;
- chemicals circulate through the heat exchanger and clean the walls;
- the spent reagent with dirt pours out.
The heat exchanger must be cleaned regularly, at least once a year. However, this procedure can be repeated more often if the quality of the coolant is very low. If ordinary tap water is poured into the system, then it is very simple to determine this: observe how quickly scale forms on the kettle. Hard water “suffers” from this deficiency.
Characteristic signs of scale formation
Signs of scale in boilers are:
- reduction in maximum heating temperature,
- increased energy consumption,
- overheating of external surfaces,
- overheating protection triggered,
- occurrence of breakdowns: heating element burnout, boiler depressurization,
- different temperatures of radiators,
- the appearance of extraneous noise.
One of the signs of the presence of scale in boilers is a decrease in the maximum heating temperature
How to clean
Many ordinary people who decide to flush the heat exchanger with their own hands, as a rule, ask two questions. How to clean this device? How to clean it? First, let's tell you what chemicals are used to flush the heat exchanger.
Cleaning products
Therefore, you need to be very careful when choosing a cleaning agent. First of all, you need to consider the following factors:
- degree of heat exchanger contamination;
- how the reagent will affect the material from which the heat exchanger is made.
At home, it is most advisable to use the following chemicals to flush this boiler element:
- citric acid, which is a fairly effective descaling agent;
- sulfamic and adipic acids are practical for regular washing of the heat exchanger, when the contamination is not so significant;
- hydrochloric acid is intended to remove fairly strong scale, but when using it, it is worth taking into account the properties of the heat exchanger material;
- gels that dissolve in water - they are not as aggressive as acidic reagents, but no less effective.
Washing methods
To clean this structural element of the boiler from scale with your own hands, it is optimal to use the following two methods:
- mechanical;
- chemical.
The essence of the mechanical method is as follows:
- the gas boiler is disconnected from the gas supply and power supply;
- the heat exchanger is disconnected from all lines and carefully removed;
- the device is soaked for 3–7 hours (depending on the degree of contamination) in a container with a low concentration acid solution;
- the heat exchanger is thoroughly washed with running water and installed in place.
Expert advice: when rinsing with running water, the device can be gently tapped with a mallet at the same time to increase the cleaning effect.
Here
The procedure for this is as follows:
- two pipes of the device are disconnected from the general boiler system;
- a booster hose is connected to one of them, through which liquid for flushing will be supplied;
- We also attach a hose to the second branch pipe, from which the reagent will flow out to the booster - thus, the cleaning agent will circulate between the heat exchanger and the booster;
- After cleaning, the spent chemical solution is drained and then washed well with water.
Specialist's note: to successfully clean the heat exchanger from scale using a booster, flushing should be done several times.
gas boiler cleaning
Watch the video in which an experienced user clearly explains the features of flushing the heat exchanger of a gas boiler:
Chemical Reagent Basics
Rinse the boiler using solutions based on hydrochloric, sulfuric, phosphoric or nitric acid: they have high oxidizing properties. To prepare such a product, it is necessary to dilute the acid in a certain proportion with water.
Both dismountable and in-place chemical cleaning of the boiler from scale is carried out in several stages. At each of them, additional doses of the reagent will be administered. This is due to the consumption of acid: having reacted with substances that make up the scale, the acid loses its basic oxidizing properties.
Washing methods
Chemical
Chemical flushing of heating systems is used in two cases:
When it is necessary to restore the functionality of the heating system of an apartment building, which has been in operation for several decades. In combination with the inevitable siltation, the overgrowth of steel pipes leads to a catastrophic drop in efficiency during this time.
The actual flushing consists of pouring a special reagent into the heating circuit instead of water. This product is a solution of alkali (most often sodium hydroxide) or acid (phosphoric, orthophosphoric, and so on).
Then a special pump in the circuit ensures continuous circulation for several hours. Then the heating system flushing fluid is drained and the system is pressure tested before being put back into operation.
This device is a reagent tank and a pump in one housing.
The cost of the cleaning product starts from 5-6 thousand rubles for 25 liters. Housing maintenance rules prohibit disposing of used solution down the drain; however, if there is no other way of disposal, solutions for their neutralization are sold along with washing compounds.
Hydropneumatic flushing
This technology for flushing the heating system has been used for many decades in the Russian (and previously in the Union) housing and communal services and has long shown its effectiveness. But only and exclusively if performed correctly.
The instructions for flushing the heating system are quite simple: the circuit is discharged into the sewer system, first from the supply to the return, then vice versa. In this case, air is pumped into the water flow by a high-power pneumatic pump. The pulp, passing along the entire contour, partially destroys the scale and removes silt.
The heating system flushing scheme used in practice in housing and communal services is as follows:
- We close the house valve on the return pipeline.
- We connect the compressor to the supply metering valve after the house valve.
- We open the reset on the return line.
- When the pressure in the ballast tank of the compressor reaches 6 kgf/cm2, open the valve to which it is connected.
- We close off groups of risers one by one so that no more than a dozen are open at any given time. In this case, flushing heating risers and heating devices connected to them will be most effective.
The duration of flushing can be easily controlled by visually assessing the degree of contamination of the water being discharged. As soon as it has become transparent, we begin to wash a new group of risers.
After all the risers are washed, we switch the heating to reset in the opposite direction:
- We close the discharge and the valve to which the compressor is connected.
- We close the house valve on the supply and open it on the return.
- We open the reset from the supply. We connect the compressor to the metering valve on the return pipeline and open it.
Next, washing the groups of risers is repeated, but with the pulp flow in the opposite direction.
Causes and consequences of clogging
To answer this question, you need to know which boiler is in the house: energy-dependent or mechanical.
Energy-dependent ones are in turn divided into:
- gas processing;
- electrical installations;
- solid fuel and liquid fuel equipment;
- combined models.
Volatile boilers may turn off for a number of reasons:
- voltage drops and surges in power lines
- lack of electricity
- factory settings failure.
Often used in homes: AOGV, Zhukovsky boiler, gas “Ochag”, Lemax, Signal, Conord .
How to clean a chimney pipe in a private house: a detailed analysis of cleaning methods, tips, prices. Do-it-yourself furnace: drawing, operation diagram. Read here.
Connection to the city water supply network: documents, prices:
https://klimatlab.com/vodosnabzhenie/vodoprovod/podklyuchenie-k-gorodskoj-seti-v-chastnom-dome.html
Mechanical ones may work intermittently due to wind flow entering the chimney. In addition, due to insufficient oxygen, the flame may go out. You should pay attention to the hood (cheaper models do not always have it).
If the chimney becomes dirty, modern devices have a warning system that will notify you of problems and the need for cleaning.
What stages do we go through when cleaning industrial boilers?
Hydrodynamic boiler flushing has a mandatory 9 stages. Here they are.
1. Draining water from the boiler
2. Opening the hatches of the upper and lower drums
3. Dismantling the steam separator
4. Cleaning of each boiler tube by a specialist located inside the upper drum
5. Final washing of the upper drum.
6. Cleaning screen and convection pipes
7. Opening and washing the collector
8. Washing the lower drum
9. Removing sludge and scale from the lower drum.