Due to the evaporation and condensation of the refrigerant in a closed circuit, the thermal energy of the air is selected and released into the environment. This is the operating principle of any refrigeration machine. The physical state and other parameters of the working substance are constantly changing. But most ordinary users are interested in only one characteristic - freon pressure in the air conditioner.
The rationale is clear: many owners of private houses and apartments want to service the split system themselves, filling the refrigerant in the simplest way found on the Internet. We will reveal the essence of the method in 3 stages - the theoretical part, diagnostics and instructions for refueling.
Preparing to refill the air conditioner
Refueling a split system requires certain preparation, which is carried out in several stages outlined below:
- The first step is to completely drain the split system. To do this, you will need to blow out the air conditioner with freon or nitrogen. Freon is used if the installation was initially carried out correctly. It is located in the outdoor block, due to which purge is realized.
- Among other things, you need to check the split system for leaks. To do this, the pressure is usually increased. If it is broken, it is necessary to understand where exactly the problem exists. The location is located using ultraviolet radiation.
- When the above conditions are met, it is necessary to remove all air from the pipeline by resorting to vacuum.
- You need to find out how much freon is required to refill the air conditioning system. Typically, all data on the required amount of refrigerant and its type is indicated on the air conditioner, but must also be written in the documents attached to the system.
Freon is used to purify the air conditioner before refilling.
Basic safety when working with R404a gas
- If there is a significant leak, R404a gas displaces air, which can pose a health hazard to people and animals in small, unventilated spaces. This is due to the fact that the components of R404a gas are heavier than air;
- If the freon gets on the skin, then in order to avoid frostbite, it is necessary to rinse the affected areas with plenty of warm water. In case of contact with the mucous membrane of the eye, it is necessary to rinse with water for a long time and immediately seek emergency medical help;
Brief inhalation of freon is safe for humans. But with prolonged exposure, the gas displaces air from the lungs, and this is fraught with such human conditions as loss of consciousness, disorientation, suffocation, drug intoxication, cardiac arrhythmia, and even death. The victim first of all needs to be provided with fresh air and, as a resuscitation measure, ventilation of the lungs using the mouth-to-mouth method. It is also necessary to seek specialized medical help.
Thus, the R-404a freon we described in this article is currently considered one of the safest for people and the environment, easy to use, efficient and inexpensive.
Application, use
Freon R134a is a one-component gas. If there is a leak, it does not require a complete refill. Equipment operating on this refrigerant can be refilled without loss of operating efficiency. This is a big advantage compared to air conditioning and refrigeration systems based on R410a, R407c, R404. Freon R134a is also used as a component of all effective substitutes for freon R22.
R134a refrigerant is non-toxic; it is used in the production of inhalers, aerosols, and capsules for freezing injuries. By following basic safety and use rules, negative effects on human health can be avoided.
The molar mass of tetrafluoroethane is 102.03 g/mol, air – 29 g/mol. When there is a leak, the gas falls down and accumulates at floor level, in the basement. This property of HFC-134a prevents it from entering the lungs under normal conditions.
Freon 134 is not compatible with mineral oils. When they come into contact, foaming may occur, oil may enter the system, and deposit on its walls. This leads to the formation of narrowed areas and blockages. Equipment using this refrigerant must be filled with PAG or POE synthetic oil.
Toxicity of freon r410a and harm to human health
If the refrigerant comes into contact with the skin, it degreases it. This may cause itching and irritation. When freon r410a enters in a liquid state, it evaporates. This leads to cooling of the skin, possibly frostbite.
The toxicity of freon r410a has been tested on animals. Throughout their lives, male rats breathed air laced with R410a refrigerant. An increased risk of developing fibrosarcomas of the salivary glands has been noted.
Fibrosarcoma is a malignant tumor that is formed from fibrous connective tissue cells.
No acute effects of poisoning or intoxication were recorded. When the concentration of gas in the air increases, it displaces oxygen. Negative consequences occur due to its deficiency, and not due to the toxicity of the r410a refrigerant. What may happen:
- Dizziness and loss of coordination;
- Increased blood pressure and pulse rate;
- Increased frequency and depth of breathing, shortness of breath;
- Arrhythmia (with acute lack of oxygen).
Charging technology based on refrigerant weight
The essence of the method is to completely replace freon - the old gas must be released into the atmosphere, and fresh freon must be filled in instead. For beginners, this is the most acceptable option - only an experienced technician is able to determine the remaining refrigerant in the system and accurately refill the missing amount. We will describe other download methods below.
Connecting the manifold to the ports of the external module
We present instructions on how to charge an air conditioner with freon using a scale:
- Drain the old refrigerant into the atmosphere in any convenient way - through an unscrewed tube or spool of the service port. Release the gas slowly so as not to lose oil. While emptying, use the hexagon to open both taps hidden under the protective nuts.
- Close the taps and connect the left hose of the pressure gauge station (blue) to the spool. Make sure the manifold valves are also closed. Connection diagram for vacuum
- Connect the yellow middle hose to the vacuum pump fitting and start the unit. Open the left low pressure valve (on the left in the diagram) and watch the vacuum gauge - the arrow should fall below zero and show a value of minus 1 bar. Also open the service port valves.
- Evacuate the freon circuit for 20 minutes. After stopping the pump, wait half an hour while watching the pressure gauge. If the needle moves back to zero, look for a leak.
- Switch the hose from the pump to the cylinder, close the left valve of the manifold. Open the tank valve a few turns and purge the hose with freon. The operation is simple: open the right valve of the station (high pressure) for 1 second. The filling vessel is connected with the same hose as the vacuum pump
- Place the cylinder on the scale properly and reset the display to zero. Open the left manifold valve again and monitor the decrease in gas mass. When the display shows the required amount of refrigerant, close the tap.
- Close both valves on the service ports, disconnect the pipe from the spool and check the split system for operation.
Standard R410 refrigerant container must be inverted
During operation, it is important not to confuse the sequence of operations and not accidentally open the filled circuit. The minimum evacuation time is 20 minutes; during this period, the pump will remove not only air from the system, but also moisture that can harm the compressor
How to refill an air conditioner with R410a freon, watch the video:
Watch this video on YouTube
Freon pressure table
Freon R410a pressure parameters on the suction side
Freon R22 pressure parameters on the suction side
Indoor temperature readings are for dry/wet bulb
But remember that high-quality diagnostics can only be carried out by a specialist who can not only connect a pressure gauge station to the required valve, but also has a good understanding of the structure and specifics of the refrigeration cycle. Many people, without possessing these skills and knowledge, as well as additional tools, such as a clamp tester, draw conclusions about the lack of freon only by the pressure in the system. Very often (especially in cold weather) this leads to the appearance of excess pressure and, subsequently, the death of the compressor.
All household split systems are supplied with refrigerant already pumped into them. If it suddenly turns out that there is a leak, then before refueling, you must find the cause of the leak, eliminate it, and only then refuel. Otherwise, the work will be done in vain and everything will happen again.
Freon R22 - consists of one component, therefore it is easier to use for refilling air conditioners in case of leakage. It can be pumped into the system without the use of electronic scales, using only a pressure gauge station and an electronic thermometer. Since freon R22 is recognized as harmful to the environment and the ozone layer, its use is gradually being phased out. In the European Union countries, this type of refrigerant has been banned since 2010. At the moment, household air conditioners are supplied to the Russian Federation only using the safer and more modern R410A freon, and in the near future equipment will begin to be supplied using the new R32 freon.
Attention: systems operating on R410 freon can be refueled only in very rare cases, and only a competent specialist can determine this. Mostly, refilling with R410a freon occurs when the length of the freon line is increased during installation, and is done by adding refrigerant strictly by weight for each meter of line that exceeds the standard, the weight is indicated in the installation instructions for the system
In cases of R410a freon leakage, air conditioners should be refilled by weight, having first removed all old freon from the system. This is due to the fact that R410a consists of two components, and in the event of a leak, one component, having a higher density, squeezes out the other, disturbing the proportion of the components, as a result of which the refrigerant loses its thermodynamic properties.
Refilling process with freon R410a.
If the “air conditioner” simply “threw” a pressure gauge unit onto the service valve and started filling the air conditioner with R410a freon without electronic scales, you should know that the result will be a call to another technician, and possibly a failure of the system.
Refilling the air conditioner is a very responsible procedure that can only be entrusted to a qualified specialist!
If you want to carry out professional diagnostics and refueling of your air conditioner, I recommend contacting our partner, who kindly provides a 15% discount on all work and materials to any buyer of our store*
*Affiliate discount is provided based on the purchase invoice
freon pressure table r22; freon pressure table r410; freon pressure table R407; freon pressure table R32
Source
R22 reference information
CF 2 CIH
DIFLUOROCHLOROMETHANE CF 2 C 1 H
(freon 22, R22, HCFC 22, freon R22)
A colorless gas with a faint odor of trichloromethane.
- Relative molecular weight 86.468
- Melting point, ℃ -157.4 [21]
- Boiling point, ℃ -40.85
- Critical temperature, ℃ 96.13
- Critical pressure, MPa 4.986
- Critical density, kg/m3 512.8
Physical properties
Vapor pressure, density and surface tension on the liquid-vapor equilibrium line
| t, ℃ | p, MPa | ᵨ´, kg/m3 | ᵨ´´, kg/m3 | σ, mN/m |
| -120 | 0,00023 | 1621 | 0,0156 | 31,7 |
| -110 | 0,00073 | 1595 | 0,0466 | 29,9 |
| -100 | 0,0020 | 1569 | 0,1198 | 28,1 |
| -95 | 0,0031 | 1556 | 0,1833 | 27,2 |
| -90 | 0,0048 | 1543 | 0,2726 | 26,3 |
| -85 | 0,0071 | 1530 | 0,3954 | 25,5 |
| -80 | 0,0103 | 1517 | 0,5603 | 24,6 |
| -75 | 0,0147 | 1504 | 0,7774 | 23,7 |
| -70 | 0,0204 | 1490 | 1,058 | 22,9 |
| -65 | 0,0279 | 1477 | 1,415 | 22,0 |
| -60 | 0,0375 | 1463 | 1,863 | 21,1 |
| -55 | 0,0495 | 1449 | 2,417 | 20,3 |
| -50 | 0,0645 | 1435 | 3,092 | 19,5 |
| -45 | 0,0829 | 1421 | 3,908 | 18,6 |
| -40 | 0,1053 | 1406 | 4,884 | 17,8 |
| -35 | 0,1321 | 1392 | 6,040 | 17,0 |
| -30 | 0,1640 | 1377 | 7,398 | 16,3 |
| -25 | 0,0216 | 1362 | 8,983 | 15,4 |
| -20 | 0,2455 | 1347 | 10,82 | 14,6 |
| -15 | 0,2964 | 1331 | 12,93 | 13,8 |
| -10 | 0,3550 | 1315 | 15,36 | 13,1 |
| -5 | 0,4220 | 1299 | 18,13 | 12,3 |
| 0 | 0,4981 | 1282 | 21,28 | 11,6 |
| 5 | 0,5842 | 1265 | 24,84 | 10,8 |
| 10 | 0,6809 | 1248 | 28,87 | 10,1 |
| 15 | 0,7892 | 1230 | 33,42 | 9,37 |
| 20 | 0,9097 | 1211 | 38,53 | 8,66 |
| 25 | 1,044 | 1192 | 44,29 | 7,95 |
| 30 | 1,191 | 1172 | 50,76 | 7,26 |
| 35 | 1,354 | 1151 | 58,04 | 6,58 |
| 40 | 1,533 | 1130 | 66,25 | 5,92 |
| 45 | 1,728 | 1107 | 75,51 | 5,27 |
| 50 | 1,942 | 1083 | 86,02 | 4,64 |
| 55 | 2,174 | 1058 | 97,98 | 4,02 |
| 60 | 2,427 | 1031 | 111,7 | 3,42 |
| 65 | 2,700 | 1002 | 127,6 | 2,84 |
| 70 | 2,997 | 970,2 | 146,3 | 2,29 |
| 75 | 3,378 | 934,8 | 168,7 | 1,76 |
| 80 | 3,664 | 894,1 | 196,2 | 1,26 |
| 85 | 4,038 | 845,1 | 232,0 | 0,80 |
| 90 | 4,442 | 780,3 | 283,1 | 0,38 |
| 95 | 4,881 | 597,7 | 439,3 | 0,05 |
Caloric properties on the liquid-vapor equilibrium line
| t, ℃ | r, kJ/kg | h´, kJ/kg | h´´, kJ/kg | s´, kJ/(kg K) | s´´, kJ/(kg K) | с´р, kJ/(kg K) | с´´р, kJ/(kg K) |
| -120 | 281,1 | 368,3 | 649,3 | 0,3689 | 2,2041 | 1,072 | 0,470 |
| -115 | 278,1 | 373,6 | 651,7 | 0,4033 | 2,1615 | 1,071 | 0,476 |
| -110 | 275,1 | 379,0 | 654,0 | 0,4366 | 2,1226 | 1,070 | 0,483 |
| -105 | 272,1 | 384,3 | 656,4 | 0,4689 | 2,0871 | 1,070 | 0,490 |
| -100 | 269,1 | 389,7 | 658,8 | 0,5003 | 2,0547 | 1,070 | 0,498 |
| -95 | 266,2 | 395,0 | 661,3 | 0,5307 | 2,0250 | 1,070 | 0,506 |
| -90 | 263,3 | 400,4 | 663,7 | 0,5604 | 1,9979 | 1,070 | 0,514 |
| -85 | 260,4 | 405,8 | 666,1 | 0,5892 | 1,9730 | 1,072 | 0,522 |
| -80 | 257,4 | 411,1 | 668,5 | 0,6173 | 1,9501 | 1,073 | 0,531 |
| -75 | 254,5 | 416,5 | 671,0 | 0,6447 | 1,9291 | 1,075 | 0,540 |
| -70 | 251,5 | 421,9 | 673,4 | 0,6716 | 1,9098 | 1,078 | 0,550 |
| -65 | 248,5 | 427,3 | 675,8 | 0,6978 | 1,8920 | 1,081 | 0,560 |
| -60 | 245,5 | 432,7 | 678,2 | 0,7236 | 1,8755 | 1,085 | 0,571 |
| -55 | 242,5 | 438,1 | 680,6 | 0,7487 | 1,8603 | 1,089 | 0,582 |
| -50 | 239,4 | 443,6 | 683,0 | 0,7735 | 1,8463 | 1,094 | 0,592 |
| -45 | 236,3 | 449,1 | 685,3 | 0,7977 | 1,8332 | 1,099 | 0,606 |
| -40 | 233,0 | 454,6 | 687,6 | 0,8276 | 1,8211 | 1,104 | 0,619 |
| -35 | 229,8 | 460,2 | 689,9 | 0,8450 | 1,8099 | 1,110 | 0,633 |
| -30 | 226,4 | 465,7 | 692,2 | 0,8681 | 1,7994 | 1,116 | 0,648 |
| -25 | 223,0 | 471,3 | 694,4 | 0,8908 | 1,7896 | 1,123 | 0,663 |
| -20 | 219,5 | 477,0 | 696,5 | 0,9132 | 1,7803 | 1,130 | 0,679 |
| -15 | 215,9 | 482,7 | 698,6 | 0,9353 | 1,7717 | 1,138 | 0,696 |
| -10 | 212,2 | 488,4 | 700,6 | 0,9571 | 1,7635 | 1,147 | 0,714 |
| -5 | 208,4 | 494,2 | 702,6 | 0,9787 | 1,7558 | 1,157 | 0,734 |
| 0 | 204,4 | 500,0 | 704,4 | 1,0000 | 1,7484 | 1,167 | 0,754 |
| 5 | 200,3 | 505,9 | 706,2 | 1,0211 | 1,7414 | 1,180 | 0,776 |
| 10 | 196,1 | 511,8 | 707,9 | 1,0420 | 1,7346 | 1,193 | 0,801 |
| 15 | 191,7 | 517,8 | 709,5 | 1,0628 | 1,7280 | 1,208 | 0,827 |
| 20 | 187,1 | 523,9 | 711,0 | 1,0834 | 1,7216 | 1,226 | 0,856 |
| 25 | 182,3 | 530,1 | 712,4 | 1,1039 | 1,7154 | 1,246 | 0,888 |
| 30 | 177,3 | 536,4 | 713,7 | 1,1244 | 1,7091 | 1,269 | 0,924 |
| 35 | 172,0 | 542,8 | 714,8 | 1,1448 | 1,7029 | 1,297 | 0,964 |
| 40 | 166,4 | 549,3 | 715,7 | 1,1653 | 1,6966 | 1,330 | 1,010 |
| 45 | 160,3 | 556,0 | 716,4 | 1,1859 | 1,6902 | 1,369 | 1,063 |
| 50 | 154,1 | 562,8 | 716,9 | 1,2067 | 1,6835 | 1,416 | 1,126 |
| 55 | 147,3 | 569,9 | 717,2 | 1,2278 | 1,6765 | 1,474 | 1,203 |
| 60 | 139,9 | 577,2 | 717,1 | 1,2492 | 1,6690 | 1,546 | 1,299 |
| 65 | 131,8 | 584,9 | 716,6 | 1,2710 | 1,6607 | 1,639 | 1,424 |
| 70 | 122,8 | 592,8 | 715,7 | 1,2936 | 1,6516 | 1,764 | 1,594 |
| 75 | 112,7 | 601,4 | 714,0 | 1,3172 | 1,6410 | 1,940 | 1,843 |
| 80 | 101,1 | 610,5 | 711,6 | 1,3422 | 1,6284 | 2,215 | 2,245 |
| 85 | 87,0 | 620,6 | 707,6 | 1,3694 | 1,6123 | 2,720 | 3,008 |
| 90 | 68,7 | 632,4 | 701,2 | 1,4009 | 1,5902 | 4,025 | 5,009 |
Caloric properties in the single-phase region
| p, MPa | h, kJ/kg | s, kJ/(kg K) | avg, kJ/(kg K) | h, kJ/kg | s, kJ/(kg K) | avg, kJ/(kg K) |
| Isotherm -80 ℃ | Isotherm -60 ℃ | |||||
| 0,01 | 668,56 | 1,9533 | 0,531 | 679,38 | 2,0066 | 0,552 |
| 0,1 | 411,15 | 0,6172 | 1,073 | 432,72 | 0,7234 | 1,085 |
| 0,5 | 411,33 | 0,6168 | 1,073 | 432,88 | 0,7229 | 1,084 |
| 1,0 | 411,55 | 0,6162 | 1,072 | 433,09 | 0,7223 | 1,083 |
| 2,0 | 411,98 | 0,6151 | 1,071 | 433,50 | 0,7211 | 1,082 |
| 5,0 | 413,30 | 0,6117 | 1,068 | 434,74 | 0,7173 | 1,077 |
| 10,0 | 415,51 | — | 1,064 | 436,84 | 0,7113 | 1,071 |
| Isotherm -40 ℃ | Isotherm -20 ℃ | |||||
| 0,01 | 690,64 | 2,0571 | 0,575 | 702,37 | 2,1053 | |
| 0,1 | 687,82 | 1,8267 | 0,617 | 700,23 | 1,8777 | 0,626 |
| 0,5 | 454,75 | 0,8210 | 1,103 | 477,06 | 0,9128 | 1,130 |
| 1,0 | 454,93 | 0,8203 | 1,102 | 477,21 | 0,9119 | 1,128 |
| 2,0 | 455,30 | 0,8188 | 1,100 | 477,53 | 0,9102 | 1,124 |
| 5,0 | 456,44 | 0,8146 | 1,093 | 478,50 | 0,9053 | 1,114 |
| 10,0 | 458,38 | 0,8078 | 1,083 | 480,21 | 0,8976 | 1,100 |
| 15,0 | 460,37 | 0,8014 | 1,075 | 482,00 | 0,8904 | 1,088 |
| 20,0 | 462,40 | 0,7952 | 1,068 | 483,87 | 0,8836 | 1,079 |
| Isotherm 0℃ | Isotherm 20 ℃ | |||||
| 0,01 | 714,57 | 2,1517 | 0,622 | 727,24 | 2,1964 | 0,645 |
| 0,1 | 712,89 | 1,9258 | 0,641 | 725,88 | 1,9717 | 0,659 |
| 0,5 | 500,00 | 1,000 | 1,168 | 719,22 | 1,8004 | 0,733 |
| 1,0 | 500,11 | 0,9990 | 1,164 | 523,94 | 1,0831 | 1,225 |
| 2,0 | 500,33 | 0,9969 | 1,158 | 524,00 | 1,0805 | 1,214 |
| 5,0 | 501,05 | 0,9911 | 1,143 | 524,30 | 1,0732 | 1,186 |
| 10,0 | 502,41 | 0,9820 | 1,121 | 525,13 | 1,0623 | 1,153 |
| 15,0 | 503,92 | 0,9737 | 1,105 | 526,24 | 1,0526 | 1,129 |
| 20,0 | 505,57 | 0,9660 | 1,092 | 527,57 | 1,0438 | 1,110 |
| Isotherm 40℃ | Isotherm 60℃ | |||||
| 0,01 | 740,38 | 2,2398 | 0,668 | 753,98 | 2,2819 | 0,691 |
| 0,1 | 739,24 | 2,0158 | 0,678 | 753,01 | 2,0584 | 0,698 |
| 0,5 | 733,84 | 1,8486 | 0,730 | 748,49 | 1,8939 | 0,736 |
| 1,0 | 726,05 | 1,7634 | 0,825 | 742,24 | 1,8136 | 0,799 |
| 2,0 | 549,19 | 1,1636 | 1,319 | 726,50 | 1,106 | 1,041 |
| 5,0 | 548,71 | 1,1537 | 1,262 | 575,26 | 1,2358 | 1,410 |
| 10,0 | 548,65 | 1,1399 | 1,204 | 573,54 | 1,2169 | 1,292 |
| 15,0 | 549,17 | 1,1282 | 1,168 | 573,12 | 1,2023 | 1,233 |
| 20,0 | 550,06 | 1,1180 | 1,142 | 573,40 | 1,1902 | 1,196 |
| Isotherm 80℃ | Isotherm 100℃ | |||||
| 0,01 | 768,01 | 2,3228 | 0,713 | 782,48 | 2,3626 | 0,734 |
| 0,1 | 767,17 | 2,0997 | 0,719 | 781,75 | 2,1398 | 0,739 |
| 0,5 | 763,31 | 1,9371 | 0,747 | 778,39 | 1,9798 | 0,761 |
| 1,0 | 758,12 | 1,8598 | 0,791 | 773,96 | 1,9035 | 0,794 |
| 2,0 | 746,02 | 1,7675 | 0,929 | 764,08 | 1,8173 | 0,884 |
| 5,0 | 606,49 | 1,3268 | 1,786 | 708,34 | 1,6044 | 3,527 |
| 10,0 | 600,70 | 1,2960 | 1,433 | 630,00 | 1,3767 | 1,553 |
| 15,0 | 598,70 | 1,2769 | 1,329 | 625,02 | 1,3494 | 1,343 |
| 20,0 | 598,08 | 1,2621 | 1,274 | 623,02 | 1,3309 | 1,259 |
| Isotherm 120℃ | Isotherm 140℃ | |||||
| 0,01 | 797,37 | 2,4015 | 0,754 | 812,65 | 2,4394 | 0,774 |
| 0,1 | 796,71 | 2,1789 | 0,758 | 712,07 | 2,2170 | 0,777 |
| 0,5 | 793,76 | 2,0188 | 0,776 | 809,43 | 2,0577 | 0,792 |
| 1,0 | 789,90 | 1,9451 | 0,801 | 806,03 | 1,9851 | 0,812 |
| 2,0 | 781,54 | 1,8629 | 0,865 | 798,78 | 1,9056 | 0,860 |
| 5,0 | 747,18 | 1,7062 | 1,399 | 772,10 | 1,7681 | 1,141 |
| 10,0 | 664,20 | 1,4660 | 1,909 | 706,21 | 1,5701 | 2,167 |
| 15,0 | 652,78 | 1,4218 | 1,437 | 682,65 | 1,4959 | 1,550 |
| 20,0 | 648,68 | 1,3978 | 1,307 | 675,36 | 1,4640 | 1,362 |
| Isotherm 160℃ | Isotherm 180℃ | |||||
| 0,01 | 828,33 | 2,4764 | 0,793 | 844,38 | 2,5127 | 0,811 |
| 0,1 | 827,80 | 2,2542 | 0,796 | 843,90 | 2,2905 | 0,814 |
| 0,5 | 825,43 | 2,0955 | 0,808 | 841,75 | 2,1323 | 0,824 |
| 1,0 | 822,39 | 2,0238 | 0,824 | 839,00 | 2,0613 | 0,838 |
| 2,0 | 815,99 | 1,9463 | 0,862 | 833,28 | 1,9853 | 0,868 |
| 5,0 | 793,80 | 1,8194 | 1,043 | 814,14 | 1,8653 | 0,996 |
| 10,0 | 745,70 | 1,6635 | 1,737 | 776,57 | 1,7333 | 1,392 |
| 15,0 | 714,48 | 1,5712 | 1,614 | 746,20 | 1,6428 | 1,537 |
| 20,0 | 703,11 | 1,5296 | 1,410 | 731,56 | 1,5938 | 1,426 |
| Isotherm 200℃ | Isotherm 250℃ | |||||
| 0,01 | 860,78 | 2,5481 | 0,829 | 903,25 | 2,6334 | 0,869 |
| 0,1 | 860,35 | 2,3260 | 0,831 | 902,90 | 2,4115 | 0,871 |
| 0,5 | 858,39 | 2,1682 | 0,840 | 901,31 | 2,2545 | 0,877 |
| 1,0 | 855,89 | 2,0977 | 0,851 | 899,30 | 2,1849 | 0,885 |
| 2,0 | 850,71 | 2,0230 | 0,876 | 895,15 | 2,1122 | 0,902 |
| 5,0 | 775,55 | 1,7062 | 1,399 | 838,90 | 1,8336 | 1,170 |
| 10,0 | 802,55 | 1,7894 | 1,225 | 859,17 | 1,9033 | 1,075 |
| 15,0 | 833,81 | 1,9078 | 0,974 | 881,98 | 2,0046 | 0,960 |
| 20,0 | 759,80 | 1,6548 | 1,389 | 824,64 | 1,7852 | 1,210 |
Density in the single-phase region ρ, kg/m3
| p, MPa | t, ℃ | ||||||||||
| -40 | -20 | 0 | 20 | 40 | 60 | 80 | 100 | 140 | 200 | 250 | |
| 0,05 | 2,271 | 2,081 | 1,923 | 1,787 | 1,671 | 1,569 | 1,478 | 1,398 | 1,262 | 1,100 | 0,995 |
| 0,1 | 4,629 | 4,220 | 3,885 | 3,603 | 3,362 | 3,153 | 2,969 | 2,806 | 2,529 | 2,204 | 1,992 |
| 0,5 | 1407 | 1347 | 1282 | |19,33 | 17,74 | 16,45 | 15,36 | 14,43 | 12,89 | 11,15 | 10,04 |
| 1,0 | 1408 | 1349 | 1284 | 1212 | |38,48 | 34,96 | 32,21 | 29,97 | 26,45 | 22,65 | 20,28 |
| 2,0 | 1410 | 1352 | 1288 | 1217 | 1134 | |82,54 | 72,40 | 65,45 | 55,93 | 46,73 | 41,39 |
| 3,0 | 1412 | 1353 | 1291 | 1222 | 1141 | 1040 | |129,3 | 109,9 | 89,32 | 72,43 | 63,35 |
| 4,0 | 1414 | 1356 | 1295 | 1227 | 1149 | 1053 | 908,2 | 172,3 | 128,0 | 99,91 | 86,20 |
| 5,0 | 1417 | 1360 | 1298 | 1231 | 1155 | 1065 | 939,6 | 298,5 | 173,8 | 129,4 | 109,9 |
| 10,0 | 1427 | 1372 | 1315 | 1253 | 1185 | 1110 | 1023 | 916,3 | 580,3 | 309,5 | 242,3 |
| 20,0 | 1427 | 1396 | 1343 | 1289 | 1232 | 1172 | 1108 | 1040 | 891,2 | 655,1 | 517,1 |
Temperature coefficient of volumetric expansion α·103, 1/Ƙ
| p, MPa | t, ℃ | |||||||||
| -40 | -20 | 0 | 20 | 40 | 60 | 80 | 100 | 140 | 200 | |
| 0,05 | 4,533 | 4,118 | 3,802 | 3,511 | 3,265 | 2,054 | 2,870 | 2,709 | 2,439 | 2,125 |
| 0,1 | 4,801 | 4,298 | 3,949 | 3,615 | 3,339 | 3,107 | 2,910 | 2,739 | 2,458 | 2,137 |
| 0,5 | 2,125 | 2,314 | 2,789 | |4,624 | 4,031 | 3,595 | 3,263 | 3,003 | 2,019 | 2,223 |
| 1,0 | 2,116 | 2,299 | 2,742 | 6,698 | |5,277 | 4,406 | 3,820 | 3,402 | 2,848 | 2,358 |
| 2,0 | 2,097 | 2,272 | 2,690 | 3,158 | 4,096 | |7,621 | 5,632 | 4,556 | 3,421 | 2,633 |
| 3,0 | 2,079 | 2,246 | 2,641 | 3,069 | 3,900 | 5,758 | |10,24 | 6,654 | 4,205 | 2,943 |
| 4,0 | 2,062 | 2,221 | 2,594 | 2,986 | 3,728 | 5,237 | 11,24 | 11,63 | 5,310 | 3,289 |
| 5,0 | 2,045 | 2,196 | 2,548 | 2,908 | 3,576 | 4,836 | 8,419 | 42,00 | 6,920 | 3,675 |
| 10,0 | 1,968 | 2,086 | 2,347 | 2,584 | 3,011 | 3,667 | 4,465 | 6,494 | 16,29 | 6,003 |
| 20,0 | 1,838 | 1,913 | 2,034 | 2,123 | 2,333 | 2,655 | 3,024 | 3,385 | 4,239 | 5,508 |
Viscosity and thermal conductivity on the liquid-vapor equilibrium line
| t, ℃ | η´, μPa s | η´´, µPa s | ν´, mm2/s | ν´´, mm2/s | λ´, mW/(m K) | λ´´, mW/(m K) |
| -100 | 860 | 7,34 | 0,548 | 61,3 | 153,0 | 2,21 |
| -95 | 770 | 7,57 | 0,495 | 41,3 | 149,5 | 2,58 |
| -90 | 700 | 7,80 | 0,454 | 28,6 | 146,5 | 2,94 |
| -85 | 642 | 8,03 | 0,420 | 20,3 | 143,0 | 3,30 |
| -80 | 594 | 8,26 | 0,392 | 14,7 | 139,5 | 3,67 |
| -75 | 547 | 8,49 | 0,364 | 10,9 | 136,5 | 4,03 |
| -70 | 506 | 8,73 | 0,340 | 8,25 | 133,0 | 4,40 |
| -65 | 472 | 8,97 | 0,320 | 6,33 | 130,0 | 4,78 |
| -60 | 438 | 9,21 | 0,299 | 4,94 | 127,5 | 5,15 |
| -55 | 408 | 9,45 | 0,281 | 3,91 | 124,5 | 5,53 |
| -50 | 382 | 9,70 | 0,266 | 3,14 | 121,5 | 5,90 |
| -45 | 358 | 9,95 | 0,252 | 2,55 | 118,5 | 6,27 |
| -40 | 336 | 10,2 | 0,239 | 2,09 | 116,0 | 6,65 |
| -35 | 316 | 10,4 | 0,227 | 1,72 | 113,0 | 7,00 |
| -30 | 295 | 10,7 | 0,214 | 1,45 | 110,5 | 7,40 |
| -25 | 276 | 10,9 | 0,202 | 1,21 | 108,0 | 7,75 |
| -20 | 260 | 11,2 | 0,193 | 1,03 | 105,5 | 8,10 |
| -15 | 247 | 11,4 | 0,186 | 0,882 | 103,0 | 8,50 |
| -15 | 247 | 11,4 | 0,186 | 0,882 | 103,0 | 8,50 |
| -5 | 221 | 11,9 | 0,170 | 0,656 | 98,0 | 9,30 |
| 0 | 210 | 12,2 | 0,164 | 0,573 | 95,5 | 9,75 |
| 5 | 199 | 12,5 | 0,157 | 0,503 | 93,0 | 10,2 |
| 10 | 188 | 12,8 | 0,151 | 0,443 | 91,0 | 10,6 |
| 15 | 178 | 13,1 | 0,145 | 0,392 | 88,5 | 11,1 |
| 20 | 170 | 13,4 | 0,140 | 0,348 | 86,0 | 11,6 |
| 25 | 158 | 13,7 | 0,133 | 0,309 | 84,0 | 12,1 |
| 30 | 150 | 14,1 | 0,128 | 0,278 | 82,5 | 12,7 |
| 35 | 141 | 14,4 | 0,123 | 0,248 | 80,5 | 13,2 |
| 40 | 133 | 14,8 | 0,118 | 0,223 | 78,5 | 13,8 |
| 45 | 126 | 15,2 | 0,114 | 0,201 | 76,5 | 14,5 |
| 50 | 118 | 15,8 | 0,109 | 0,184 | 74,5 | 15,2 |
| 55 | 112 | 16,3 | 0,106 | 0,166 | 72,5 | 16,0 |
| 60 | 106 | 17,0 | 0,103 | 0,152 | 70,5 | 16,8 |
| 65 | 101 | 17,8 | 0,101 | 0,139 | 68,0 | 17,7 |
| 70 | 94,7 | 18,7 | 0,0976 | 0,128 | 66,0 | 18,7 |
| 75 | 88,0 | 19,7 | 0,0942 | 0,117 | 63,0 | 20,0 |
| 80 | 80,9 | 21,0 | 0,0905 | 0,107 | 60,0 | 21,4 |
| 85 | 73,1 | 22,7 | 0,0865 | 0,0982 | 57,0 | 23,0 |
| 90 | 63,9 | 25,7 | 0,0815 | 0,0907 | 53,5 | 25,0 |
Viscosity and thermal conductivity in the single-phase region
| p, MPa | η, μPa s | λ´, mW/(m K) | η, μPa s | λ´, mW/(m K) |
| Isotherm -100 ℃ | Isotherm -40 ℃ | |||
| 0,1 | 860,4 | 152 | 9,97 | — |
| 1,0 | 865,4 | — | 337,4 | 119 |
| 5,0 | 888,0 | 153 | 348,8 | 121 |
| 10,0 | 916,8 | — | 363,0 | 122 |
| 20,0 | 977,4 | — | 391,3 | 126 |
| 60,0 | 1322,3 | — | 509,5 | 140 |
| Isotherm 0℃ | Isotherm 50℃ | |||
| 0,1 | 11,9 | 9,1 | 13,9 | 12,3 |
| 1,0 | 210,1 | 96,2 | 14,5 | 13,4 |
| 5,0 | 219,4 | 99,4 | 129,0 | 78,0 |
| 10,0 | 231,5 | 103 | 143,2 | 83,2 |
| 20,0 | 253,2 | 106 | 164,9 | 88,9 |
| 60,0 | 342,3 | — | 233,7 | — |
| Isotherm 100℃ | Isotherm 130℃ | |||
| 0,1 | 15,9 | 15,4 | 17,0 | 17,3 |
| 1,0 | 16,4 | 16,4 | 17,4 | 18,2 |
| 5,0 | 26,5 | 26,3 | 22,6 | 23,8 |
| 10,0 | 85,2 | 62,8 | 52,0 | 47,4 |
| 20,0 | 112,1 | 73,5 | 89,1 | 66,0 |
| Isotherm 160℃ | Isotherm 200℃ | |||
| 0,1 | 18,1 | 19,2 | 19,5 | 21,8 |
| 1,0 | 18,5 | 20,0 | 19,8 | 22,4 |
| 5,0 | 22,3 | 24,5 | 22,8 | 26,1 |
| 10,0 | 35,2 | 35,9 | 30,5 | 33,0 |
| 20,0 | 70,8 | 58,9 | 52,7 | 50,7 |
Other physical properties
| Heat of formation standard ΔН°298, kJ/mol | -475 |
| Temperature of allotropic transformation, ℃ | -214,15 |
| Heat of allotropic transformation, kJ/mol | 0,016 |
| Heat of fusion, kJ/mol | 4,12 |
| Heat of evaporation at boiling point, kJ/mol | 20,19 |
| Adiabatic index at 25 ℃ and 0.1 MPa | 1,184 |
| Dipole moment, C m | 4.7 10-30 (1.41D) |
| Breakdown voltage: | |
| steam relative to nitrogen at 25 ℃ and 0.1 MPa | 1,27 |
| Liquid, MV/m, or kV/mm | 120 |
| Specific electrical conductivity at 22 ℃, S/m: | |
| liquid | 1,2·10-6 |
| steam at 0.1 MPa | 4,8·10-11 |
| The dielectric constant: | |
| liquid at 24℃ | 6,11 |
| Steam at 25.4 ℃ and 0.5 MPa | 1,0034 |
| Steam at 25.4℃ and 0.1 MPa | 1,0069 |
| Refractive index | 1,267 |
Solubility
Mass solubility of difluorochloromethane in water at a partial pressure of 0.101 MPa, %:
| 0 ℃ | 0,778 | 50 ℃ | 0,162 |
| 10 ℃ | 0,519 | 60 ℃ | 0,132 |
| 20 ℃ | 0,365 | 70 ℃ | 0,110 |
| 30 ℃ | 0,269 | 80 ℃ | 0,09 |
| 40 ℃ | 0,206 |
and water in difluorochloromethane:
| -40 ℃ | 0,012 | 10 ℃ | 0,082 |
| -30 ℃ | 0,019 | 20 ℃ | 0,111 |
| -20 ℃ | 0,028 | 30 ℃ | 0,147 |
| -10 ℃ | 0,042 | 40 ℃ | 0,191 |
| 0 ℃ | 0,059 |
Molar solubility of difluorochloromethane in organic solvents at 20 ℃ and partial pressure 0.101 MPa,%:
| Dicumylmethane | 10,5 | Methyl salicylate | 7,1 |
| Oleic acid | 11,9 | Dimethyl phthalate | 12,1 |
| Benzyl acetate | 11,3 | Diethyl phthalate | 15,4 |
| Dibutyl sebacate | 23,8 | Dibutyl phthalate | 18,3 |
| Dioctyl sebacate | 25,8 | Dioctyl phthalate | 23,0 |
| Methyl benzoate | 10,5 | Didecyl phthalate | 21,0 |
| Propyl benzoate | 12,4 | Dicapryl phthalate | 23,0 |
| Betyl benzoate | 13,4 | Dimethylformamide | 14,0 |
With water it forms a crystalline hydrate of composition CF2C1H · 8.4H2O with upper point parameters of 16.25 ℃, 0.77 MPa.
Environmental characteristics and fire safety
ODP=0.050; HGWP=0.34; GWP=1700. MPCr.z=3000 mg/m3; MPCv=10 mg/l. Hazard class 4.
When in contact with flames on hot surfaces, it decomposes to form highly toxic products.
Non-flammable gas.
Thermal stability
Thermal decomposition at a contact time of 1-10 s begins in a tube made of steel 12Х18Н10Т at 280 ℃, from nickel N-1 at 380 ℃.
Corrosive to metals and non-metals
Metal materials, resistant at 50 ℃ (corrosion rate no more than 0.005 mm/year): steels 12Х13, 14Х17Н2, 12Х18Н9Т, 12Х18Н10Т, 15Х18Н12С4ТУ, nickel N-2, NP-2, Monel metal NMZhMts 28-2.5-1, 5, titanium VT-1-1M, aluminum AD1, aluminum alloy AMg6, copper M3, brass L90.
Non-metallic materials, resistant at 15-30 ℃ (swelling no more than 15% by weight): fluoroplastics 4, 40, 3, vinyl plastic, polyethylene, polyisobutylene PBSG, textolite I-1, rubber SKF-32 with tube carbon black, ebonite 1751, impregnated graphite, arzamite 5, epoxy resin, PON paronite, fiberglass, faolite.
Chemical properties
- Halogenation. At a temperature of 400-600 ℃ in the gas phase in bulk or on a catalyst, it reacts with chlorine and bromine:
CF2CIH + CI2 → CF2CI2 + HCI;CF2CIH + Br2 → CF2CIBr + HBr.
- Hydrolysis. In the presence of metals, it reacts very slowly with water:
CF2CIH + 2H2O → HCOOH + 2HF + HCIHydrolyzes with alkalis and alcoholates, forming formates:
CF2CIH + 4NaOH → HCOONa + 2NaF + NaCI + 2H20.
- Alkylation.
At high temperatures, it reacts in bulk with tetrachlorethylene, forming predominantly 3,3-difluorotetrachloropropylene: CF2CIH + CCI2 = CCI2 → 500-600℃ → CF2CICCI = CCI2 + HCI. - Interaction with fluoroalcohols. In the presence of alkali metal hydroxides, it forms fluoroethers:
CF2CIH + CF3CH2OH + NaOH → P-body → CF3CH2OCF2H + NaCI + H2O;CF2CIH + CF3CH2OH + KOH → Δ; 70-95℃ → CF3CH2OCF2H + KCI + H2O;
CF2CIH + CF2HCF2CH2OH + NaOH → (CH2CH2CH2)2O; 6-20℃ → CF2HCF2CH2OCF2H + NaCI + H2O.
- Disproportionation.
At elevated temperatures in the presence of a catalyst (chloride or activated aluminum oxide), it disproportionates: 5CF2CIN → 150-250℃ → 3CF3H + CFCI2H + CCI3H. - Pyrolysis.
At high temperatures in volume it undergoes thermal decomposition with the formation of tetrafluoroethylene: 2CF2CIH → 650-800℃ → CF2 + 2HCI.
Synthesis methods
- Fluorination of trichloromethane with mercury difluoride:
CCI3H + 2HgF2 → CF2CIH + CI2 + 2HgF. - Fluorination of trichloromethane with antimony trifluoride in the presence of antimony pentachloride:
CCI3H + SbF3 → SbCI5; 100℃; 5.7 MPa → CF2CIH + SbFCI2. - Fluorination of trichloromethane with hydrogen fluoride in the presence of antimony trichloride or pentachloride:
CCI3H + 2HF → SbCI3 or SbCI5 → CF2CIH + 2HCI. - Gas-phase catalytic fluorination of trichloromethane with hydrogen fluoride in the presence of metal oxides and halides:
2CCI3H + 3HF → CrOF; 130-180℃; 1MPa → CF2CIH + CFCI2H + 3HCI. - Reduction of difluorodichloromethane with hydrogen at high temperature:
CF2CI2 + H2 → 685℃ → CF2CIH + CF2H2 + other products.
Laboratory method of obtaining
Interaction of trichloromethane and hydrogen fluoride in the presence of antimony pentachloride. The same equipment is used as for the synthesis of difluorodichloromethane.
400 g (1.34 mol) of antimony pentachloride, 720 g (6 mol) of trichloromethane and 360 g (18 mol) of cold hydrogen fluoride are loaded into a cooled reactor through a tube. Heat the reactor in a water bath at 80 ℃. Within 6.5 hours, the pressure in the reactor reaches about 2.3 MPa. Open the valve and release gaseous products into the absorption and condensing part of the system at such a speed that the resulting hydrogen chloride has time to be absorbed by water. The condensate is distilled on a low-temperature column, collecting the main fraction from -40 to -36 ℃.
345 g (4 mol) of difluorochloromethane are obtained. The trichloromethane yield is 66.5%.
Industrial production
In industry, it is obtained by liquid-phase fluorination of trichloromethane with hydrogen fluoride in the presence of a catalyst - antimony pentachloride.
The obtaining process consists of the following main stages:
- fluoridation of carbon dioxide;
- purification of synthesis gas from hydrogen chloride and hydrogen fluoride;
- compression, drying and condensation of difluorochloromethane and organofluorine impurities;
- separation of difluorochloromethane by rectification.
Technical diagram
Difluorochloromethane and hydrogen fluoride in a molar ratio of 1:2 are fed into the reactor. The process is carried out at a temperature of 60-90 ℃ and a pressure of 0.55-0.85 MPa. The synthesis gas after the reflux condenser enters a graphite plate neutralization column, irrigated with a 10% calcium carbonate solution, for final neutralization of acidity. Raw gas is collected in a gas tank, from where it is supplied to the compensation unit through a drying column with active aluminum oxide using a compressor. Condensation of the raw material takes place at a pressure of 1.35 MPa. The separation of difluorochloromethane and fluorodichloromethane is carried out in three continuous distillation columns, where low-boiling impurities (air, trifluoromethane) are stripped off and commercial difluorochloromethane is separated.
By-products and methods of their disposal
Hydrochloric acid (22-27%) - 3.4 tons per 1 ton of product and a mixture of hydrochloric and hydrofluoric acids - 1 ton. per 1 ton of product; are produced in accordance with technical specifications and are used in the national economy.
Gas blow-offs from the distillation column in the amount of 5-6 kg. per 1 ton of product containing up to 80% trifluoromethane is sent to extract the latter.
The bottom residue (up to 4 kg per 1 ton of product) is sent for combustion.
Technical requirements for the finished product
| Volume fraction of difluorochloromethane, %, not less | 99,9 |
| Volume fraction of impurities determined by chromatographic method, %, no more | 0,1 |
| Mass fraction of non-volatile residue, %, no more | 0,001 |
| Mass fraction of water, %, no more | 0,001 |
Transportation and storage
It is poured into railway tanks, as well as into cylinders with a capacity of 32 to 130 dm3, into containers and other vessels designed for a pressure of 2 MPa. Fill factor 1.0 kg. product per 1 dm3 of vessel capacity.
Transported by any type of transport. Store in warehouses that provide protection from sunlight.
Application
Refrigerant for obtaining temperatures up to -40℃ in the 1st stage or up to -60℃ in the 2nd stage of refrigeration machines, in industrial and domestic air conditioners, component of mixed refrigerants, low-temperature propellant, steam generator for the production of foam plastics. Widely used for the production of fluoromonomers (tetrafluoroethylene, hexafluoropropene) and other organofluorine products.
Download certificate .PDF
Download MSDS (English).PDF
SOURCE: Industrial Organofluorine Products, 2nd edition, revised and expanded
Checking the valves of the outdoor unit
Another way to find out that there is no or little refrigerant in the air conditioner is to check the valves of the outdoor unit. They are located at the bottom, on the right side and can be covered with a protective casing (see photo). This option works best in the warm season.
Location of air conditioner service valves.
Before checking, turn on the air conditioner at full power and set the minimum temperature. Let it run for 5-10 minutes
Then pay attention to the valves. If condensation has accumulated on them, they have cooled down - everything is in order, there is refrigerant in the system
If there is no condensation on the service valves and their temperature has not changed, the freon has completely left the air conditioner. If frost or ice has formed on the surface of the valves, there is not enough refrigerant in the system. Ice forms for the same reason as in the outdoor unit.
Latest publications
Consequences of contact with refrigerant
It must be said right away that severe freon poisoning is rare, but if you encounter a severe form of intoxication, the complications are quite serious.
- Respiratory failure. The main cause of this condition is pulmonary edema.
- Local skin lesions. Direct contact with liquid freon may result in a chemical burn. The worst option is tissue necrosis.
- Liver and kidney failure. These organs are the first to take the blow, and the consequence for them is either partial or complete loss of function.
- Heart failure. This complication can occur if toxic breakdown products of freon enter the bloodstream under the influence of high temperatures (during a fire).
Extremely severe poisoning and lack of first aid to the victim are conditions that can lead to death. The main prerequisite is pulmonary edema, in which case the work of paired organs is completely blocked.
Specifications
In terms of physical properties, a mixture of two hydrofluorocarbons is close to azeotropic. During phase transitions, its temperature glide is minimal, almost equal to 0. This means that both components simultaneously evaporate and condense. Freon R 410a has high cooling capacity. Improved performance makes it possible to reduce the size of climate control equipment and refrigeration units. The refrigerant is non-toxic and fireproof; it does not ignite in air.
Physical characteristics of freon r410a
| Characteristics | Units | Meaning |
| Molecular mass | 72,6 | |
| Boiling temperature | °C | -52 |
| Saturated vapor density at boiling | Kg/m3 | 4 |
| Critical temperature | °C | 72 |
| Critical pressure | MPa | 4,93 |
| Temperature drift | °C | 0,15 |
| Heat of vaporization | KJ/kg | 264.3 |
| Specific heat capacity of steam | Btu/lb*°F | 0,17 |
| Ozone depletion factor | ||
| Global Warming Potential (GWP) | 1890 | |
| ASHRAE Safety Group | A1/A1 |
High global warming potential is a disadvantage of the connection. The ejection effect is similar to R22. The system is refilled only in the liquid phase. Transportation and storage are carried out in pink cylinders that can withstand a pressure of 48 bar. Containers are filled to 75% weight.
Characteristics of r410a refrigerant on the saturation line
| T, | Davl. | Density | Enthalp. | Entropy | Davl. | Density | Enthalp. | Entropy | Heat |
| °C | sat. | kg/cub.m | kJ/kg | kJ/(kg*K) | sat. | kg/cub.m | kJ/kg | kJ/(kg*K) | steam generation (kJ/kg) |
| -50 | 1,123 | 1339,761 | 131,4 | 0,726 | 1,122 | 4,526 | 401,5 | 1,936 | 270,1 |
| -45 | 1,417 | 1325,036 | 137,8 | 0,754 | 1,415 | 5,616 | 404,6 | 1,924 | 266,8 |
| -40 | 1,77 | 1309,941 | 144,2 | 0,782 | 1,767 | 6,909 | 407,5 | 1,913 | 263,4 |
| -35 | 2,191 | 1294,45 | 150,7 | 0,809 | 2,187 | 8,435 | 410,5 | 1,902 | 259,8 |
| -30 | 2,689 | 1278,534 | 157,3 | 0,837 | 2,683 | 10,224 | 413,3 | 1,891 | 256 |
| -25 | 3,273 | 1262,162 | 164 | 0,864 | 3,265 | 12,312 | 416,1 | 1,882 | 252 |
| -20 | 3,954 | 1245,297 | 170,9 | 0,891 | 3,944 | 14,738 | 418,8 | 1,872 | 247,8 |
| -15 | 4,743 | 1227,897 | 177,9 | 0,918 | 4,73 | 17,546 | 421,3 | 1,863 | 243,4 |
| -10 | 5,651 | 1209,914 | 185,1 | 0,945 | 5,635 | 20,785 | 423,8 | 1,854 | 238,7 |
| -5 | 6,69 | 1191,292 | 192,5 | 0,973 | 6,67 | 24,511 | 426,1 | 1,846 | 233,6 |
| 0 | 7,872 | 1171,968 | 200 | 1 | 7,849 | 28,79 | 428,3 | 1,837 | 228,3 |
| 5 | 9,211 | 1151,863 | 207,7 | 1,028 | 9,184 | 33,696 | 430,2 | 1,829 | 222,5 |
| 10 | 10,719 | 1130,887 | 215,7 | 1,055 | 10,688 | 39,317 | 432 | 1,821 | 216,3 |
| 15 | 12,41 | 1108,928 | 223,9 | 1,084 | 12,375 | 45,759 | 433,6 | 1,812 | 209,6 |
| 20 | 14,299 | 1085,849 | 232,5 | 1,112 | 14,26 | 53,149 | 434,8 | 1,803 | 202,4 |
| 25 | 16,399 | 1061,481 | 241,3 | 1,141 | 16,357 | 61,643 | 435,8 | 1,794 | 194,5 |
| 30 | 18,725 | 1035,603 | 250,5 | 1,171 | 18,681 | 71,44 | 436,4 | 1,785 | 185,9 |
| 35 | 21,293 | 1007,926 | 260,2 | 1,202 | 21,247 | 82,798 | 436,6 | 1,774 | 176,4 |
| 40 | 24,116 | 978,057 | 270,4 | 1,233 | 24,07 | 96,062 | 436,2 | 1,763 | 165,9 |
| 45 | 27,211 | 945,435 | 281,2 | 1,266 | 27,165 | 111,722 | 435,2 | 1,75 | 154 |
| 50 | 30,592 | 909,218 | 292,8 | 1,301 | 30,549 | 130,504 | 433,4 | 1,736 | 140,6 |
In this article we have given the characteristics of R-410a freon, its pressure and temperature tables. They also talked about the history of origin and features. You learned about the toxicity of freon r410a and its effect on humans. We hope the publication was useful. Save it to your wall and don’t forget to share with friends and colleagues.
Do you want to get help from a master, a specialist in this field? Go to the professional search portal Pro . This is a completely free service where you will find a professional who will solve your problem. You do not pay for posting an ad, views, or choosing a contractor. If you are a master of your craft, then register on Pro and receive a flow of clients. Your profit is just one click away!
How often should the system be charged with refrigerant?
Preventive refilling of freon is carried out 1-2 times a year, since absolute tightness does not exist. In accordance with regulatory documents, refrigerant losses of 5-8% during the year are allowed.
The second reason is a decrease in operating efficiency when the freon pressure in the air conditioner drops due to leakage through leaks in roller and other connections. If there is a malfunction, you can hear a continuous hum from the compressor. In this case, a full cycle of measures is carried out to eliminate the leak and fill it with refrigerant.
Before refueling the air conditioner at home, you should make sure that you have all the necessary equipment listed in the list:
- a refrigerant bottle of the exact brand your cooler requires;
- dry nitrogen cylinder;
- set of hoses with threaded connections;
- manifold;
- electronic balance;
- vacuum pump for air conditioner.
Smell and color
Many people, when encountering refrigerant for the first time, ask the question: “ what color is freon?” In its normal state and without special additives, this gas has no color. For technical needs, it can be specially painted in special shades and smelled for easier identification in the event of a leak. However, as practice shows, in the event of a leak, freon is painted either in the color of the damaged unit (as in refrigerators), or may have the smell of burnt rubber, as is the case in car air conditioners.
How often and in what cases should the air conditioner be refilled?
The air conditioner needs to be recharged in the following cases:
- After installation or reinstallation to a new location.
- After repair, if the freon lines were disconnected during the process.
- If there is a leak from the circuit. Almost always the air conditioner has to be recharged due to a leak.
- Every 2 years (frequency is approximate, if the system is installed correctly, then less often is possible). On average, over 1 year of operation, about 8% of the refrigerant volume is lost.
You can tell when it’s time for a replacement by the quality of the device’s operation.
Signs of a leak are:
- frost appears on the outdoor unit;
- the room cools (or heats up) more slowly than before (at approximately the same outside temperature); At the same time, the air conditioner works longer (or without interruptions at all), and it has to be loaded more;
- the inverter model may often turn off and show a fault code;
- When the air conditioner operates, an unpleasant odor (not dust) appears.
Split systems
It is impossible to accurately determine the refilling time, since it depends on the quality of installation, as well as the quality of the air conditioner itself.
It is better to focus on signs indicating a freon deficiency.
But in any case, it is better to check the presence of freon in the system at least once every two years.
It is convenient to do this in conjunction with cleaning the air conditioner, that is, to carry out service maintenance.
Mobile and window air conditioners
These types of air conditioners are assembled in a single housing and all connections are located inside and are made at the factory using the soldering method.
Such air conditioners require refilling very rarely. But their peculiarity is that they cannot be refueled—the entire system must be completely refilled.
You will need to refill the air conditioner after certain types of repairs:
- compressor replacement
- replacing a four-way valve
- replacing the filling port
- after damage to the heat exchangers - condenser or evaporator
- after damage to freon tubes
Where can I buy
The best price offers can be found from direct importers. Because they always have the goods in stock, have qualified quality control specialists on staff, and the “margin”, as a rule, is not as large as that of intermediaries.
One of these is the DIRECT importer of products Zhejiang Sanmei Chemical Industry Co.Ltd (China).
In terms of quality, the products of this manufacturer are comparable to their American and European counterparts, and in price they are much cheaper. It is best to place an order on the company’s official website (sanmei-ua.com). This is beneficial for several reasons:
- The price will definitely be cheaper than when buying in your hometown.
- Free consultation with specialists on selecting the right freon.
- All products have quality certificates.
- Any volume is available to order.
- The goods can be sent by any transport company in Ukraine (Novaya Poshta, SAT, Night Express, etc.)
If you have any doubts regarding the selection of a freon analogue, specialists will help you select it correctly. However, given the fact that Sunmei Ukraine is one of the main importers to Ukraine, most likely, the matter will not come to selection - original and high-quality freon will be offered.
Types of air conditioners
Manufacturers produce all types of air conditioners, investing heavily in their business. As a result, a modern consumer can choose any model according to any parameters.
Air conditioners split systems
Split type devices are great for small rooms.
There are two types of such devices: separation systems and multi-dividing systems. Wall-mounted split system units consist of two blocks: a small internal unit and a large external module.
The external device contains the loudest devices. A multi split system is formed by combining several indoor units into a single outdoor module. This allows you to optimally preserve the design of the house.
Ceiling type air conditioners
In rooms with a large area, as a rule, units for installation on the ceiling are chosen. Their advantage is that the cooled air is evenly distributed horizontally throughout the room without directly affecting people.
A massive ceiling-type air conditioner is almost invisible, and it is indispensable when extensive air flow is needed to the most remote parts of the room, while the jet length in some models reaches up to 55 meters.
There are also duct and cassette ceiling air conditioners. In this case, the first devices are completely hidden behind a suspended ceiling or in a channel, and the second type - cassette blocks have the form of ceiling tiles measuring 600x600 mm.
Split system
Although the disconnect system consists of indoor and outdoor modules, its operating principle is no different from any other type of domestic ceiling air conditioner.
The housing of the external unit itself contains a heat exchanger, a fan and a compressor. Additional elements of the split system are a dryer, expansion valve and connecting pipes.
And also to connect the unit to the electrical network, it contains the necessary starting and control devices.
Industrial air conditioners
Such devices are developed to serve areas of more than 350 meters and therefore they have a number of features, thereby differing from household air conditioners. The design of precision equipment may vary.
Multizone devices. These VRF and VRV air conditioning units include up to 64 indoor modules and up to three outdoor units. In total, they are located on communications up to 300 meters long.
It is possible to set a separate temperature for each indoor module and provide its own microclimate in each room. The error in the set temperature is only 0.05 degrees.
"Chiller fan coil." Devices with this system differ in that not freon is used inside the circuit, but water or antifreeze. The central refrigeration unit is called a “chiller”, and the heat exchange elements are called “fan coils”.
Chiller-fan coil circuit 2
The advantage of such a unit is that the distance between these components can be any, since water flows through ordinary pipes.
Central and rooftop air conditioners. These devices are varied in their action. They are used in the form of heat exchange units, fans, air purifiers and humidifiers.
It is called central because the air mass is processed in the indoor unit and then moves through pipes through the rooms. Installation of air conditioners of this type and installation of communications is particularly complex and requires an external source of cold.
If possible, it is better to choose roof monoblocks, which are easier to install.
What is freon R22
Difluorochloromethane or R22 refrigerant was until recently used as a working fluid in 90% of air conditioners. Due to its physical characteristics, it is an excellent refrigerant. Inside the systems, freon changes its state of aggregation, taking away heat and producing cold. To perform the functions of a refrigerant, the substance must have a low boiling point, as well as the resulting condensation pressure and vapor volume. Freon R22 meets the requirements, its boiling point is -40.8°C and its pressure is 4.986 MPa.
The refrigerant can be charged into domestic and industrial air conditioning units. It is compatible with mineral and alkylbenzene oils. Freon R22 has a low chlorine content, its ozone depletion potential is ODP = 0.05, global warming potential is GWP = 1700. The substance is a transition refrigerant that replaces R12 in all applications. Its cold performance is 60% higher.
The refrigerant is suitable for low temperature cooling systems with piston and screw compressors:
- household, industrial and automobile air conditioners;
- refrigeration units, including automotive and marine;
- cryogenic equipment.
Difluorochloromethane is used as a low-temperature propellant in aerosol cans, a foam converter, and a component for the production of fluoromonomers. Refrigerant R22 is used in stage I and II refrigeration machines to obtain temperatures of -40° and -60°C, respectively. It is a component of a mixture of refrigerants.
A common option for selling gas is a metal cylinder with a valve and a safety valve.
Impact on the ozone layer
The effect of freon on the ozone layer is 20 times less than that of the previously used freons R11 and R12. The gas belongs to the group chlorofluorocarbons (HCFC). Refrigerants have a harmful effect on the ozone layer and increase the greenhouse effect. After being used in climate control equipment, aerosols, and refrigerators, they enter the atmosphere. They decompose under the influence of solar ultraviolet radiation. Free components of freons react with ozone, provoking its decay. According to the UN Montreal Protocol, the production and use of HCFCs is reduced and gradually phased out. China has not joined the global agreement; refrigeration equipment and air conditioners manufactured in the country run on R22 freon.
Refrigerant advantages:
- Freon R22 is stable, non-toxic and explosion-proof.
- The low discharge temperature during compression in the compressor prevents overheating of the mechanism.
- The refrigerant has excellent thermophysical and thermodynamic characteristics.
- Chemical inertness to most structural materials (copper, brass, nickel, steel).
- Freon 22 is offered at an affordable price, cheaper than its R407c counterpart.
- It contains one component, which simplifies the refilling of air conditioners in case of leakage.
- The absence of a temperature glide does not change the composition of the substance in the liquid and gas phases.
Where is freon used?
Today, freon is used not only to cool spaces, but also as a constituent element in the production of polystyrene foam and as an indicator of the integrity and tightness of a vacuum system. Experts identify 3 main areas of freon use:
- refrigerant for refrigeration equipment;
- in the production of perfumes and medical purposes;
- one of the components of modern fire extinguishers.
It is most popular in the production of refrigerators and climate systems (air conditioners).
Types of refrigerants
All modern air conditioning equipment runs on freon. As it condenses inside the device, it releases heat, and when it evaporates, on the contrary, it absorbs it. Thanks to this unique property, it can effectively operate the air conditioning system. If you turn on the air conditioner, the freon will evaporate and take heat out of the room. Thus, the air is cooled. Over time, with repeated use of the split system, freon gradually evaporates from the air conditioner. Thus, the system needs to be refueled from time to time. The substance evaporates from the internal circuit of the equipment, at the junction of the outdoor unit of the air conditioner with the indoor unit.
Freon is an absolutely safe gas that is sold in cylinders in specialized stores. There is a wide range of modern air conditioning refill products on sale. The choice of refrigerant must be made based on knowledge of the brand of air conditioner.
Freon R-410A is a new generation refrigerant that is absolutely safe for the environment and also has a positive effect on the performance of the air conditioner due to the use of higher pressure. And this, in turn, greatly saves energy.
Freon R-407C is intended for refilling industrial air conditioning equipment. When such freon leaks, the light components evaporate first. The disadvantages include the fact that the air conditioner charged with it cannot be refilled later. You will have to completely remove the freon from the equipment and only then carry out a full refill.
Refilling the split system with freon is carried out approximately once or twice a year. But, if you just purchased it, then several years will most likely pass before the time when you need to refuel. The time between subsequent refuelings varies depending on the brand of equipment you have installed. You can find out more precisely from the instruction manual, or by consulting the specialist who installed your air conditioner.
Finally, we strongly recommend not to skimp on refilling your air conditioner. Do not buy cheap refrigerant, which in most cases will be of poor quality. Monitor the condition of your climate system, and if you notice any signs that the equipment does not have enough refrigerant, then urgently refill it. Then the device will work stably and efficiently. Now you know how to pump freon into the air conditioner correctly, which will undoubtedly extend the life of your split system.
Oil for 410 freon
The equipment requires synthetic polyester (POE) compressor oil to operate. The viscosity is selected depending on the equipment and operating conditions. There are several manufacturers producing lines of refrigeration oils for 410 refrigerant:
- Mobil EAL Arctic 32.46, 68.100;
- PLANETELF ACD 32.46, 68.100;
- Suniso SL 32, 46,68,100;
- Emkarate RL 32, 46, 68, 85,100, 170, 220;
- Biltzer BSE 32, 55, 170, 170L.
What is freon R-404a
Freons (freons) are colorless and odorless substances. They come in gas or liquid form. A distinctive feature of freons is that they are poorly soluble in water, but are easily affected by various organic solvents. Most freons are non-flammable, although there are exceptions, for example, freon R600A. In addition, these substances boast that they are not resistant to oxidizing agents and acids.
Freon R404 (404A) is a freon that was obtained artificially in order to become an analogue of R22 and R502. As planned, R404 was supposed to not only absorb the best qualities of both freons, but also have a number of advantages according to certain criteria.
If we look at the history of freon 404A, it turns out that it is relatively young and was born in 1994. It did not immediately receive widespread demand. At first, freon 404A was used only in equipment specially designed for it, mainly large refrigeration devices intended for commercial needs.
Gradually, freon 404A began to be used in various wholesale warehouses, stores, refrigerators, and shop windows, eventually gaining its current popularity.
Of course, such a demand for freon 404A did not arise out of nowhere. It was facilitated by the advantages that this refrigerant has.
It is noteworthy that although freon 404A is not a flammable substance, it contains one - R143A. But even for freon to ignite R143A, certain conditions of temperature and pressure will be required. However, experts recommend not to tempt fate and to follow basic safety measures when working with freon 404A
What are these precautions? Freon 404A should not be mixed with air
It is important to avoid high pressures and temperatures
When working with freon 404A, it should be taken into account that it dissolves well in essential oils, but practically does not mix with mineral oils, and, what is noteworthy, this happens at a variety of temperatures.
Today, freon 404A continues to carve out its niche among refrigeration equipment and is increasingly displacing its predecessor - freon R502
It is important to take into account that when replacing the old freon with freon 404A, the oil must also be changed
History of the name
In 1928, the American chemist at General Motors Research, Thomas Midgley (1889-1944), managed to isolate and synthesize in his laboratory a chemical compound that was later named Freon. After some time, Chemical Kinetic, which was engaged in the industrial production of a new gas - Freon-12, introduced the designation of the refrigerant with the letter R (Refrigerant - cooler, refrigerant). This name became widespread and over time the full name of the refrigerants began to be written in a composite form - the manufacturer’s trademark and the generally accepted designation of the refrigerant. For example: the brand GENETRON®AZ-20 corresponds to the refrigerant R-410A, which consists of refrigerants R-32 (50%) and R-125 (50%). There is also a trademark with the same name as the chemical compound - FREON® (Freon), the main copyright holder of which was previously the American company (DuPont), and now The Chemours Company (Chemours), created on the basis of one of the divisions of DuPont . This coincidence in the name still causes confusion and controversy - whether arbitrary refrigerants can be called freon.
How to find a freon leak in a split system with your own hands?
The choice of detection method depends on the nature of the damage to the route, its length and the possibility of access to the suspected location of the freon leak. In addition, some methods require the use of special equipment and materials. Among them, the most common are the following:
- Soap solution. It is used when the location of a freon leak is theoretically known (for example, when oil stains or drips are detected in a certain section of the route) and allows you to visually determine the leak. Apply a regular soap solution or a special product in an aerosol package to the suspected site of damage.
- Immersion in water. The simplest method for detecting leaks in the refrigeration circuit, which is convenient to use if the part being tested is small in size, can be removed from the system and sealed.
- Penetrating dye. This method involves pumping a special ultraviolet dye into the cooling circuit, which, after some time, can be detected in leakage areas. Unlike a soap solution, the dye allows you to find a leak along the entire length of the route, however, the disadvantage of this method is the long wait for the result and the need for expensive equipment.
- Pressure test. Allows you to find the location of depressurization by increasing the pressure in the system (but not higher than the limits set by the manufacturer). To do this, dry nitrogen is pumped into the circuit, the pressure is increased to the maximum level, after which its value is recorded using a pressure gauge. A decrease in pressure over time indicates a leak.
- Leak detectors. Special diagnostic equipment allows you to find freon leaks with a high degree of accuracy. Currently, halide, ultrasonic and electronic leak detectors are used to test air conditioner circuits. The latter are the most effective tools because they have adjustable sensitivity levels. How to check an air conditioner for freon leaks using a leak detector? To do this, the place of possible leakage is carefully wrapped with polyethylene, then the pressure in the system is slightly increased, and after that a small slot is made in the lower part of the polyethylene, to which the device is brought.
Methods for controlling the amount of freon
When figuring out how to charge a split system with freon yourself, you should remember that the amount of refrigerant should be sufficient, but not excessive. If there is too much gas in the circuit, the operation of the device will be seriously impaired, since the refrigerant simply will not have time to evaporate. This can seriously damage the compressor.
This situation is worse for the device than if the system is missing a few grams of refrigerant. Therefore, during refueling, it is necessary to organize control of the amount of freon entering the system.
They do this in the following ways:
- by measuring the change in mass of the refrigerant cylinder;
- taking into account the pressure in the system, which must reach a certain level;
- assessing the condition of the circuit through the sight glass;
- taking into account the temperature change at the indoor unit fan.
The easiest way to control the amount of freon is to record changes in the weight of the cylinder. To do this, before refilling, place the container with the refrigerant on the scale, reset the result and observe the change in indicators when the cylinder tap is open.
As soon as its weight decreases by the required amount, refueling is stopped immediately. Of course, this method is only used to completely charge the circuit. If you just need to top up the system, you first need to find out the weight of the refrigerant that is already inside, but this is difficult to do at home.
There are professional scales designed for these purposes, but many masters make do with inexpensive household models.
The device must meet the following requirements:
- load capacity – at least 20 kg;
- scale gradation – from 100 g;
- Availability of container weighing option.
It is most convenient to use electronic scales, which make it easier to track changes in the weight of the refrigerant container.
Another available option is to bring the pressure inside the circuit to the desired level. To perform this type of filling, you will need a pressure manifold. Using this device, the pressure inside the system is assessed.
The refrigerant is supplied to the circuit in small portions, constantly checking the pressure information with the standard indicator until a match is achieved.
Before charging the system with refrigerant, it is necessary to find out why the leak occurred and then correct any problems found. Re-inspection is carried out after completion of work
A collector is quite expensive equipment that does not make sense to buy only to use once every few years. It will be useful not only at the stage of pumping freon, but also when draining and vacuuming the system. You can borrow such a device from a familiar master or rent it at a specialized point.
The sight glass method is available to professionals. It consists of observing the state of the refrigerant flow, monitoring the moment when air bubbles disappear from it. At home, the first two methods are often used.
Measuring temperature is a simple, but not very reliable method. When the fan circuit is full, the temperature should usually be about eight degrees, although there are models for which this figure is five; a deviation of a couple of degrees is allowed. The refrigerant is introduced in small portions, taking measurements periodically.
Basic data
The cooling effect of freon is formed as a result of boiling of this substance to a certain temperature point and the subsequent pressure created, as a result of which a cooling effect is created. When assessing the cooling effect (to what temperature a space can be cooled), the boiling point at atmospheric pressure and the critical boiling point are of considerable importance.
Temperature
The boiling point of freon at atmospheric pressure is an approximate value that indicates how much the chamber temperature can be lowered or what cold temperature can be achieved using minimum pressure by vacuuming. Simply put, this is the minimum cold temperature that will be observed in the cooling chamber, at minimum pressure. For example, for R22 it is 44.8°C, and for the no less popular R134a -26.5°C.
The critical temperature is the maximum temperature at which gas condensation can still be achieved. This is the temperature at which a gas can still change from a gaseous state to a liquid state. For example, for R22 it is +96°C, and for R134A – +100.6°C.
As a rule, information about gas temperatures is written on the freon cylinder itself and on the original packaging.
Pressure
The pressure created inside the cooler system plays a key role in cooling the chamber. The adiabatic coefficient (coefficient) of refrigerant vapor is used as an indicator of pressure. The pressure is taken to be the work of compression 1k and the temperature of the steam at the end of the compression procedure. Simply put, the greater the adiabatic value, the higher the value of 1k, as well as the temperature of the steam at the end of the compression process.
Freon r134a: detailed characteristics, properties, features of the refrigerant
Freon R134a (tetrafluoroethane) is a colorless gas with a boiling point of -29 °C. Due to its technical characteristics, r134a refrigerant is used in air conditioners, chillers, refrigeration equipment, polymer production, medicine and cosmetology. It is known as:
- HFC R134a;
- Freon-134;
- Refrigerant R134-a;
- Refrigerant 134a;
- Freon 134a;
- HFC 134a.
We will talk about the characteristics of freon 134, physical and chemical properties, and application. You will learn about the features of using and operating equipment using R134a. We present tables of boiling point, pressure of refrigerant r134-a, properties of saturated vapor and liquid under different conditions. Detailed technical information about the refrigerant can be found in the Tables and diagrams section.
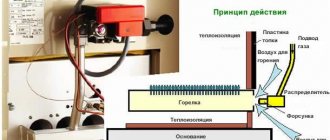
How is freon made?
On an industrial scale, freon is produced by supplying hydrogen fluoride in a gaseous state without an evaporator (i.e., without a “coil”). A homogenizer mixer is used to produce a homogeneous state of hydrogen fluoride, which subsequently becomes hydrogen fluoride in chloroform.
Next, hydrogen fluoride in chloroform enters the refrigerant synthesis reactor, a kind of “coil” in which droplets are evenly distributed throughout the entire reaction volume.
Those. In many ways, the process of producing freon is similar to the process of producing moonshine, but using specific equipment.
Properties[ | ]
Physical properties
Freons are colorless gases or odorless liquids. Well soluble in non-polar organic solvents, very poorly soluble in water and other polar solvents. Basic physical properties of methane series freons
| Chemical formula | Name | Technical designation | Melting point, °C | Boiling point, °C | Relative molecular weight |
| CFH3 | fluoromethane | R-41 | -141,8 | -79,64 | 34,033 |
| CF2H2 | difluoromethane | R-32 | -136 | -51,7 | 52,024 |
| CF3H | trifluoromethane | R-23 | -155,15 | -82,2 | 70,014 |
| CF4 | tetrafluoromethane | R-14 | -183,6 | -128,0 | 88,005 |
| CFClH2 | fluorochloromethane | R-31 | — | -9 | 68,478 |
| CF2ClH | chlorodifluoromethane | R-22 | -157,4 | -40,85 | 86,468 |
| CF3Cl | trifluorochloromethane | R-13 | -181 | -81,5 | 104,459 |
| CFCl2H | fluorodichloromethane | R-21 | -127 | 8,7 | 102,923 |
| CF2Cl2 | difluorodichloromethane | R-12 | -155,95 | -29,74 | 120,913 |
| CFCl3 | fluorotrichloromethane | R-11 | -110,45 | 23,65 | 137,368 |
| CF3Br | trifluorobromomethane | R-13B1 | -174,7 | -57,77 | 148,910 |
| CF2Br2 | difluorodibromomethane | R-12B2 | -141 | 24,2 | 209,816 |
| CF2ClBr | difluorochlorobromomethane | R-12B1 | -159,5 | -3,83 | 165,364 |
| CF2BrH | difluorobromomethane | R-22B1 | — | -15,7 | 130,920 |
| CFCl2Br | fluorodichlorobromomethane | R-11B1 | — | 51,9 | 181,819 |
| CF3I | trifluoromethane | R-13I1 | — | -22,5 | 195,911 |
Chemical properties
Freons are relatively inert chemically, so they do not burn in air, are not explosive even when in contact with an open flame, but actively interact with alkali and alkaline earth metals, pure aluminum, magnesium, and magnesium alloys. The formation of mixtures with air or oxygen under pressure and contact with metal heated above 200° C is prohibited! When freons are heated above 250 °C, very toxic products are formed, for example, phosgene COCl2, which was used as a chemical warfare agent during the First World War.
Resistant to acids and alkalis.
Rules for the digital designation of freons (freons)
According to the international standard ISO No. 817-74, the technical designation of freon (freon) consists of the letter designation R (from the word refrigerant) and the digital designation:
- the first number on the right is the number of fluorine atoms in the compound;
- the second number on the right is the number of hydrogen atoms in the compound plus one;
- the third digit from the right is the number of carbon atoms in the compound minus one (for methane compounds the zero is omitted);
- the number of chlorine atoms in a compound is found by subtracting the total number of fluorine and hydrogen atoms from the total number of atoms that can combine with carbon atoms;
- for cyclic derivatives, the letter C is placed at the beginning of the defining number;
- in the case where bromine is in place of chlorine, the letter B and a number indicating the number of bromine atoms in the molecule are placed at the end of the identifying number.
- in the case where iodine is in place of chlorine, the letter I and a number indicating the number of iodine atoms in the molecule are placed at the end of the identifying number.