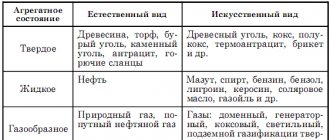
Today, there are several types of solid fuels that are used as an energy carrier. Such fuel is: wood, coal, various fuel briquettes, as well as peat. Coal is considered the best fuel that can ensure the efficiency of a furnace or boiler. Today, charcoal is widely used, as well as fossil fuels. The popular charcoal is produced only artificially, namely through wood processing, but nature itself creates fossil fuels. Both types are widely used in some industries, as well as in everyday life.
Chemical process
After entering the chamber, gradual smoldering of the firewood occurs.
This process occurs due to the presence in the firebox of a sufficient amount of oxygen gas that supports combustion. As the smoldering process occurs, a sufficient amount of heat is released and excess liquid is converted into steam. The smoke released during the reaction goes to the secondary processing compartment, where it burns completely and heat is released. A charcoal kiln performs several important functional tasks. With its help, charcoal is formed, and a comfortable temperature is maintained in the room.
But the process of obtaining such fuel is quite delicate, and with the slightest delay, complete combustion of the wood is possible. It is necessary to remove charred pieces from the oven at a certain time.
How to stay safe
But the “lobsters” don’t just eat their yeast-free bread. Along with the discovery of all kinds of dirty tricks, they also discover ways to reduce the amount of this dirty trick. Here's some advice you can glean from numerous works on this subject:
- Use stable fuel combustion. It's better to cook with gas rather than wood. Then less harmful substances will be released. Charcoal is also better to prefer to firewood.
- When grilling, smoke is one of the sources of contamination of the prepared product. If the smoke is removed without passing through the product, the benzopyrene content in the product is halved.
- Avoid dripping fat and other biological substances even onto coals. Fat burns, but not completely, hence the increased formation of pollutants. This is just that delicious smoke. Reduction of benzopyrene by almost 90%.
- Cooking in foil allows you to almost completely protect the product from contact with benzopyrene.
- Using a microwave, for example, to fully or partially cook a dish, is the best way to reduce the content of nastiness in the final dish.
- Do not overcook until a crust forms; use lower temperatures for cooking.
In general, forewarned is forearmed. It is for this reason, after listening and reading all sorts of research, that I decided to try a relative novelty on our market - a vertical barbecue.
Types and grades of coal
Coal is classified according to many parameters (geography of extraction, chemical composition), but from a “domestic” point of view, when buying coal for use in furnaces, it is enough to understand the labeling and the possibility of use in ThermoRobot.
According to the degree of coalification, three types of coal are distinguished: brown
,
stone
and
anthracite.
The following coal designation system is used:
Grade
= (
grade)
+ (
size class).
In addition to the main grades given in the table, intermediate grades of coal are also distinguished: DG (long-flame gas), GZh (gas fatty), KZh (coke fatty), PA (semi-anthracite), brown coals are also divided into groups. Coking grades of coal (G, coke, Zh, K, OS) are practically not used in thermal power engineering, since they are a scarce raw material for the coke-chemical industry. According to the size class (size of pieces, fractions), graded coal is divided into:
In addition to graded coal, there are combined fractions and screenings available for sale (PK, KO, OM, MS, SSh, MSSh, OMSSh). The size of coal is determined based on the smaller value of the finest fraction and the larger value of the largest fraction indicated in the name of the coal grade. For example, the OM fraction (M - 13–25, O - 25-50) is 13–50 mm.
In addition to the above-mentioned types of coal, you can find coal briquettes on sale, which are pressed from low-enriched coal slurry.
How coal burns
Coal consists of two flammable components: volatiles
and
solid (coke) residue
.
During the first stage of combustion, volatile substances are released; When there is an excess of oxygen, they burn quickly, producing a long flame but little heat.
After this, the coke residue burns out; the intensity of its combustion and ignition temperature depend on the degree of coalification, that is, on the type of coal (brown, hard, anthracite). The higher the degree of carbonization (the highest is for anthracite), the higher the ignition temperature and heat of combustion, but the lower the combustion intensity.
Coal grades D, G
Due to the high content of volatile substances, such coal flares up quickly and burns out quickly. Coal of these grades is available and suitable for almost all types of boilers, however, for complete combustion, this coal must be supplied in small portions so that the released volatile substances have time to completely combine with oxygen in the air. Complete combustion of coal is characterized by a yellow flame and clear flue gases; incomplete combustion of volatile substances produces a purple flame and black smoke. To effectively burn such coal, the process must be constantly monitored; this operating mode is implemented in the Termorobot automatic boiler room.
Coal grade A
It is more difficult to light, but it burns for a long time and produces much more heat. Coal can be loaded in large batches, since they burn mainly coke residue and there is no mass release of volatile substances. The blowing mode is very important, since if there is a lack of air, combustion occurs slowly, it may stop, or, on the contrary, an excessive increase in temperature, leading to heat loss and burnout of the boiler.
Everyone knows that fuel use plays a huge role in our lives. The fuel is used in almost every branch of modern industry. Fuel derived from oil is especially often used: gasoline, kerosene, diesel fuel and others. Combustible gases (methane and others) are also used.
Carbon
Carbon
Carbon is a non-metallic element of group IV of the periodic table D.I. Mendeleev, is the most important part of all organic substances in nature.
General characteristics of group IVa elements
From C to Pb (from top to bottom in the periodic table) there is an increase in: atomic radius, metallic, basic, reducing properties. Electronegativity, ionization energy, and electron affinity decrease.
Of the elements of group IVa, carbon and silicon are non-metals, germanium, tin and lead are metals.
The electronic configurations of these elements are similar, since they are in the same group (main subgroup!), the general formula ns 2 np 2:
- C - 2s 2 2p 2
- Si - 3s 2 3p 2
- Ge - 4s 2 4p 2
- Sn - 5s 2 5p 2
- Pb - 6s 2 6p 2
Natural compounds
In nature, carbon occurs in the form of the following compounds:
- Allotropic modifications - graphite, diamond, fullerene
- MgCO3 - magnesite
- CaCO3 - calcite (chalk, marble)
- CaCO3*MgCO3 - dolomite
Receipt
Carbon is obtained during the pyrolysis of hydrocarbons (pyrolysis - heating without access to oxygen). The production of carbon compounds: wood and coal is also used.
Chemical properties
When heated, carbon reacts with many non-metals: hydrogen, oxygen, fluorine.
2C + O2 → (t) 2CO (carbon monoxide is a product of incomplete oxidation of carbon, formed when there is a lack of oxygen)
C + O2 → (t) CO2 (carbon dioxide is a product of complete oxidation of carbon, formed when there is a sufficient amount of oxygen)
Reactions with metals
When heated, carbon reacts with metals, exhibiting its oxidizing properties. Let me remind you that metals can only take positive oxidation states.
Ca + C → CaC2 (calcium carbide, CO carbon = -1)
Al + C → Al4C3 (aluminum carbide, CO carbon -4)
Obviously, the degree of oxidation of carbon in combination with different metals may differ.
Carbon is a good reducing agent. With its help, the metallurgical industry copes with the task of obtaining pure metals from their oxides:
Carbon reduces not only metals from their oxides, but also nonmetals in a similar way:
SiO2 + C → (t) Si + CO
It can also reduce its own oxide:
The well-known reaction of interaction of coal with water vapor, also called gasification of coal, peat, shale, is extremely important in industry:
Reactions with acids
In reactions with acids, carbon acts as a reducing agent:
Carbon monoxide II - CO
Carbon monoxide II is a product of incomplete oxidation of carbon. Non-salt-forming oxide. This extremely dangerous substance is often formed during fires in confined spaces, or when a car is warmed up in a garage.
Dissolving in the blood, carbon monoxide (which has a 300 times greater affinity for hemoglobin than oxygen) easily outcompetes oxygen and takes its place in red blood cells. Carbon monoxide poisoning is often fatal.
In industry, carbon monoxide is produced by the reduction of carbon monoxide IV or gasification of coal (t = 1000 °C).
In the laboratory, carbon monoxide is obtained from the decomposition of formic acid in the presence of sulfuric acid:
Completely oxidizes to carbon dioxide in reaction with oxygen, reducing metal oxides.
FeO + CO → Fe + CO2
Formation of carbonyls - extremely toxic substances.
Carbon monoxide IV - CO2
Product of complete oxidation of carbon. Refers to acidic oxides, corresponds to carbonic acid H2CO3. Colorless gas, odorless.
In industry, carbon dioxide is obtained from the decomposition of limestone, during the production of alcohol, and from the alcoholic fermentation of glucose.
In laboratory conditions, the reaction of chalk (marble) with hydrochloric acid is used.
Carbon dioxide is formed when organic substances burn:
- Reaction with water
As a result of the reaction with water, unstable carbonic acid is formed, which immediately breaks down into water and carbon dioxide.
Reactions with basic oxides and bases
During reactions with bases and basic oxides, carbon dioxide forms salts of carbonic acid: medium - carbonates (with an excess of base), acidic - bicarbonates (with an excess of acidic oxide).
2KOH + CO2 → K2CO3 + H2O (base-acid oxide ratio 2:1)
KOH + CO2 → KHCO3 (base-acid oxide ratio 1:1)
When heated, it is capable of oxidizing metals to their oxides.
Zn + CO2 → (t) ZnO + CO
Pyrolysis furnace: charcoal burning temperature
Charcoal is not a fossil at all. This fuel is produced by humans in special pyrolysis furnaces. The process of obtaining it is quite simple and consists of processing wood by pyrolysis. Simply put, you need to remove all moisture from the wood.
During the entire smoldering process, a lot of heat is generated, and moisture evaporates and evaporates. The smoke that is produced is recycled in a special compartment and burns completely there, generating heat.
Stages of producing charcoal:
- The critical stage is drying;
- The most important is pyrolysis;
- Then - calcination;
- And finally - cooling.
A charcoal-fired pyrolysis furnace can heat both small and large houses
Charcoal begins to ignite at a temperature of 100 - 200 degrees, and flares up to 800 - 900. When it burns, a sufficient amount of heat is released that can warm the room.
Open mining method
The main advantage of open-pit coal mining is its relative safety. The thing is that it is used only if the depth of the rock is no more than 100 meters. In other words, a shaft is not created that could collapse during an accident. The extraction process itself is carried out according to the following procedure.
First you need to remove the top layer of soil that covers the rock. This layer is called overburden, and the method of removing it is called stripping. This procedure, depending on the type of soil, is carried out using bulldozers, draglines, rotary excavators or scrapers. After the soil layer has been removed, you can proceed to crushing the rock itself. For this, crushers, water cannons, bulldozers and other equipment are used. If the rock in a coal deposit is too dense, then in rare cases, drilling and blasting of coal is used. This mining method usually covers a fairly large area.
As for the disadvantages of the method, they are as follows:
- Firstly, causing significant harm to the environment at the mining site.
- Secondly, all the rock that is mined in this way contains a large amount of harmful impurities in its composition.
The main advantages of open-pit coal mining, in addition to safety, are high speed and cost-effectiveness.
Activated carbon
Activated carbon is a type of carbon with a high specific pore surface area, which makes it even more absorbent than wood. The raw materials used for its production are charcoal and coal, as well as coconut shells. The starting material is subjected to an activation process. Its essence is to open clogged pores using high temperature, electrolyte solutions or water vapor.
During the activation process, only the structure of the substance changes, therefore the chemical formula of activated carbon is identical to the composition of the raw materials from which it was made. The moisture content of activated carbon depends on the specific pore surface area and is usually less than 12%.
Source
Creating optimal conditions for combustion
Due to the high temperature, all internal elements of the furnace are made of special refractory bricks. Fireproof clay is used for their installation. If special conditions are created, it is quite possible to obtain a temperature in the furnace exceeding 2000 degrees. Each type of coal has its own flash point.
After reaching this indicator, it is important to maintain the ignition temperature by continuously supplying excess oxygen to the firebox
Among the disadvantages of this process, we highlight heat loss, because part of the released energy will escape through the pipe. This leads to a decrease in the temperature of the firebox. In the course of experimental studies, scientists were able to establish the optimal excess amount of oxygen for various types of fuel. Thanks to the choice of excess air, you can count on complete combustion of the fuel. As a result, you can count on minimal losses of thermal energy.
Formation and origin of coal seams
The appearance of coal on Earth dates back to the distant Paleozoic era, when the planet was still in the development stage and had a completely alien appearance to us. The formation of coal seams began approximately 360,000,000 years ago. This happened mainly in the bottom sediments of prehistoric reservoirs, where organic materials accumulated over millions of years.
Simply put, coal is the remains of the bodies of giant animals, tree trunks and other living organisms that sank to the bottom, decayed and were pressed under the water column. The formation process of deposits is quite long, and it takes at least 40,000,000 years to form a coal seam.
Comparative table of indicators
The table presents the values of the mass specific heat of combustion of liquid, solid, and gaseous fuels.
| Type of fuel | Unit change | Specific heat of combustion | ||
| MJ | kW | kcal | ||
| Firewood: oak, birch, ash, beech, hornbeam | kg | 15 | 4,2 | 2500 |
| Firewood: larch, pine, spruce | kg | 15,5 | 4,3 | 2500 |
| Brown coal | kg | 12,98 | 3,6 | 3100 |
| Coal | kg | 27,00 | 7,5 | 6450 |
| Charcoal | kg | 27,26 | 7,5 | 6510 |
| Anthracite | kg | 28,05 | 7,8 | 6700 |
| Wood pellets | kg | 17,17 | 4,7 | 4110 |
| Straw pellets | kg | 14,51 | 4,0 | 3465 |
| Sunflower pellets | kg | 18,09 | 5,0 | 4320 |
| Sawdust | kg | 8,37 | 2,3 | 2000 |
| Paper | kg | 16,62 | 4,6 | 3970 |
| Vine | kg | 14,00 | 3,9 | 3345 |
| Natural gas | m3 | 33,5 | 9,3 | 8000 |
| Liquefied gas | kg | 45,20 | 12,5 | 10800 |
| Petrol | kg | 44,00 | 12,2 | 10500 |
| Dis. fuel | kg | 43,12 | 11,9 | 10300 |
| Methane | m3 | 50,03 | 13,8 | 11950 |
| Hydrogen | m3 | 120 | 33,2 | 28700 |
| Kerosene | kg | 43.50 | 12 | 10400 |
| Fuel oil | kg | 40,61 | 11,2 | 9700 |
| Oil | kg | 44,00 | 12,2 | 10500 |
| Propane | m3 | 45,57 | 12,6 | 10885 |
| Ethylene | m3 | 48,02 | 13,3 | 11470 |
The table shows that hydrogen has the highest TST indicators of all substances, not just gaseous ones. It belongs to high-energy fuels.
The product of hydrogen combustion is ordinary water. The process does not emit furnace slag, ash, carbon dioxide and carbon dioxide, which makes the substance an environmentally friendly combustible. But it is explosive and has a low density, so this fuel is difficult to liquefy and transport.
Examples of problem solving
| Exercise | The mass fraction of chlorine in phosphorus chloride is 77.5%. Determine the simplest formula of the compound. |
| Solution | The mass fraction of element X in a molecule of the composition NX is calculated using the following formula: |
ω (X) = n × Ar (X) / M (HX) × 100%
Let's calculate the mass fraction of phosphorus in the compound:
ω(P) = 100% - ω(Cl) = 100% - 77.5% = 22.5%
Let us denote the number of moles of elements included in the compound as “x” (phosphorus) and “y” (chlorine). Then, the molar ratio will look like this (the values of relative atomic masses taken from D.I. Mendeleev’s Periodic Table are rounded to whole numbers):
x:y = ω(P)/Ar(P) : ω(Cl)/Ar(Cl);
x:y= 22.5/31 : 77.5/35.5;
x:y= 0.726 : 2.183 = 1 : 3
This means that the formula for combining phosphorus with chlorine will be PCl3. This is phosphorus(III) chloride.
| Exercise | Determine the simplest formula for the compound of potassium with manganese and oxygen, if the mass fraction of potassium is 24.7%, manganese 34.8%. |
| Solution | The mass fraction of element X in a molecule of the composition NX is calculated using the following formula: |
ω (X) = n × Ar (X) / M (HX) × 100%
Let's calculate the mass fraction of oxygen in the compound:
ω (P) = 100% - ω(K) - ω(Mn) = 100% - 24.7% - 34.8% = 40.5%
Let us denote the number of moles of elements included in the compound as “x” (potassium), “y” (manganese) and “z” (oxygen). Then, the molar ratio will look like this (the values of relative atomic masses taken from D.I. Mendeleev’s Periodic Table are rounded to whole numbers):
x:y:z = ω(K)/Ar(K) : ω(Mn)/Ar(Mn) : ω(O)/Ar(O);
x:y:z= 24.7/39 : 34.8/55 : 40.5/16;
x:y:z= 0.63:0.63:2.53 = 1:1:4
This means that the formula for the compound of potassium, manganese and oxygen will be KMnO4. This is potassium permanganate.
Source
Features of different types of fuel
Let's consider the two main, most common types of solid fuel raw materials - firewood and coal. Firewood contains a significant amount of moisture, so the moisture first evaporates, which will require a certain amount of energy. After the moisture evaporates, intense combustion of the wood begins, but, unfortunately, the process does not last long.
Therefore, to maintain it, regular addition of firewood to the firebox is required. The ignition temperature of wood is about 300°C.
In terms of the amount of heat generated and the duration of combustion, coal is superior to wood. Depending on the age of the fossil material, the mineral is divided into types:
- brown;
- stone;
- anthracite.
Brown coals
Among fossil coals, the youngest are brown coals. The fuel got its name from its brown color. This type of fuel is characterized by a large amount of volatile impurities and high moisture content - up to 40%. In this case, the amount of pure carbon can reach 70%.
Due to high humidity, brown coal has a low combustion temperature and low heat transfer. Fuel ignites at 250°C, and the combustion temperature of brown coal reaches 1900°C. The calorific value is approximately 3600 kcal/kg.
As an energy carrier, brown coal in its natural form is inferior to firewood, so it is rarely used for stoves and solid fuel units in private homes. But briquetted fuel is in steady demand.
Brown coals
Brown coal in briquettes is a fuel that has undergone special preparation. By reducing humidity, its energy efficiency increases. The heat transfer of briquetted fuel reaches 5000 kcal/kg.
Stone coals
Hard coals are older than brown coals; their deposits are located at a depth of up to 3 km. In this type of fuel, the content of pure carbon can reach 95%, and volatile impurities - up to 30%. This energy carrier contains no more than 12% moisture, which has a positive effect on the thermal efficiency of the mineral.
The combustion temperature of coal under ideal conditions reaches 2100°C, but in a heating furnace the fuel is burned at a maximum of 1000°C. The heat transfer of coal fuel is 7000 kcal/kg. It is more difficult to ignite - it requires heating up to 400°C to ignite.
Coal energy is most often used to heat residential buildings and other buildings.
Stone wall
Anthracite
The oldest solid fossil fuel, which contains practically no moisture and volatile impurities. The carbon content in anthracite exceeds 95%.
The specific heat transfer of the fuel reaches 8500 kcal/kg - this is the highest indicator among coals. Under ideal conditions, anthracite burns at 2250°C. It ignites at a temperature of at least 600°C - this is an indicator for the lowest calorie types. Ignition requires the use of wood to create the necessary heat.
Characteristics of anthracite
Anthracite is primarily an industrial fuel. Its use in a furnace or boiler is irrational and expensive. In addition to high heat transfer, the advantages of anthracite include low ash content and low smoke.
Amount of firewood for winter
One of the paradoxes of the universe: the more ordinary and familiar an object of natural origin, the more complex its mathematical description. In order to calculate the volume of a Galaxy or star, it is enough to remember a school geometry course. If someone really needs to know the exact volume of a log, it is impossible to do without differential calculus methods. Because of an astronomer's mistake, the average person is neither hot nor cold; But if the fuel reserves for the winter are incorrectly calculated, the cold in the house is ensured.
Everything seems simple: we multiply the duration of the heating season in days by the area of the house and the average daily consumption of firewood. Do not believe this simplicity, it is deceptive and requires many clarifications. In fact, it is also necessary to take into account the height of the ceiling, i.e. not area, but volume. The most interesting thing begins when it comes to the average daily consumption of firewood. This value depends on the calorie content of the fuel, the efficiency of the furnace, climatic conditions, heat loss and many other factors, including the radius of curvature of the master’s hands. The last parameter is an irrational value that can reduce heating efficiency to zero. If you set out to derive a universal formula for accurate calculations, there will be enough material for more than a dozen dissertations. It is much faster to make inquiries from neighbors or former owners of the house.
If you are planning to install a solid fuel boiler, it is easier to calculate its “appetite” - the main technical characteristics are known. The easiest way to get an approximate figure is to use formulas or online calculators posted on specialized websites.
Let's try to calculate.
As a standard, let's take a house with a total area of 150 square meters. m and insulated according to SNiP. In the coldest time, about 100 W/m² will be needed to heat the room. Let's take the average energy consumption as 50 W/m². The heating season lasts 7 months (214 days). With continuous heating we get:
150 m²•50 W/m²•24 h•214 days = 38.52 MWh, which approximately corresponds to 33 Gcal.
The specific lower heat of combustion of absolutely dry firewood is 4440 kcal/kg. During natural drying for about two years, the residual moisture content of the wood is 20%, the specific heat of combustion is 3400 kcal/kg. Let us take the efficiency of a solid fuel boiler to be 70%.
We calculate the required amount of firewood: 33000kcal•1000/3400kcal/kg/ 0.7/0.730 kg/m³≈19 m³, where 0.730 kg/m³ is the density of oak wood. In terms of maple or birch, the required value is 21.3 m³, and for pine – 26.4 m³. In practice, you may need less or more firewood, depending on the state of the house’s thermal insulation and weather conditions.
All-knowing statistics claim that to heat a small insulated log house in central Russia, 4-6 cubic meters of firewood per season is sufficient. The most economically justified supply of firewood is for two or three seasons: current + 1-2 next. In this case, you will probably have enough fuel even for the longest cold period. Another argument: firewood, like good alcohol, only gets better over time.
“God have mercy, what kind of firewood? We are civilized people, we have gas!” – this position is fundamentally wrong. Firstly, because we have the good fortune to live in the most amazing country in the world. Here, from time immemorial, two scenarios have been observed: the unlikely and the worst. Secondly, the civilized world is returning to proven and, most importantly, renewable energy sources. Firewood, straw and peat are all the same as hundreds of years ago, adjusted for modern technology.
Having a solid fuel boiler and at least a year’s supply of firewood in a gasified house is not a whim, but a completely reasonable solution. Optimism is good, but optimism backed by strategic reserves is twice as good.
You can also use our online calculator to calculate the amount of chopped wood for heating a country house, cottage or bathhouse.
Coal options
In winter, the issue of heating residential premises is especially relevant. Due to the systematic increase in the cost of coolants, people have to look for alternative options for generating thermal energy. The best way to solve this problem is to select solid fuel boilers that have optimal performance characteristics and retain heat well.
The specific heat of combustion of coal is a physical quantity that shows how much heat can be released during the complete combustion of a kilogram of fuel
In order for the boiler to operate for a long time, it is important to select the correct fuel for it. The specific heat of combustion of coal is high (22 MJ/kg), so this type of fuel is considered optimal for efficient boiler operation
The combustion temperature of charcoal is much higher, so this fuel option is an excellent alternative to conventional firewood. We also note the excellent heat transfer rate, the duration of the combustion process, and low fuel consumption. There are several types of coal, related to the specifics of mining, as well as the depth of occurrence in the bowels of the earth: hard, brown, anthracite.
Coal has an ignition temperature of 400 degrees. Moreover, the calorific value of this type of coal is quite high, so this type of fuel is widely used for heating residential premises.
Anthracite has maximum efficiency. Among the disadvantages of such fuel, we highlight its high cost. The combustion temperature of this type of coal reaches 2250 degrees. No solid fuel extracted from the bowels of the earth has such an indicator.
Burning rules
When a consumer becomes familiar with the combustion temperature of a particular coal, he needs to take into account that manufacturers indicate only those figures that are relevant for ideal conditions. Of course, it is simply impossible to recreate the necessary parameters in an ordinary household boiler or oven. Modern heat generators made of metal or brick are simply not designed for such high temperatures, since the main coolant in the system can quickly boil. That is why the combustion parameters of a particular fuel are determined by its combustion mode.
In other words, it all depends on the intensity of the air supply. Both fossil and charcoal heat a room well if the oxygen supply level reaches 100%. To restrict the air flow, you can use a special damper/gate. This approach makes it possible to create the most favorable combustion conditions for filled fuel (up to 950˚C).
If coal is used in a solid fuel boiler, then boiling of the coolant must not be allowed. The main danger is that the safety valve may simply not work, which can lead to a large explosion. In addition, a mixture of water and hot steam has a bad effect on the functionality of the circulation pump. Experts have developed two most effective methods that allow you to control the combustion process:
- Crushed or powdered fuel must enter the boiler exclusively in a dosed volume (the same scheme applies as in pellet devices).
- The main energy carrier is loaded into the firebox, after which the intensity of the air supply is adjusted.
Consequences of heating wood
A process where an area of rock is exposed to an external heat source and fire appears. It could be paper set on fire with a match, or something else. What is the combustion temperature of paper? Paper can ignite if there is an ignition source on its own, releasing light and heat.
At a temperature of 120-150°C, the wood begins to char. Self-igniting coal is formed.
When the degrees reach 250-350°C, the material will begin to thermally decompose into its components. The surface of the tree will begin to smolder, but the flame cannot be seen. Brown smoke will appear. The breed has already warmed up and is ready to move into a new stage.
Ignition
The initial stage of the combustion process in which thermochemical reactions are accelerated. Activates at temperatures of 460 degrees and above.
Additional energy is required to evaporate water. Also, thermal conductivity slows down. A massive round tree burns worse. Rectangular with a small cross-section - good. An unplaned wood surface is more likely to ignite than a smooth one.
It is also important that there is enough oxygen supplied. Otherwise you will have to wait a long time for fire
Self-ignition
The process is possible without the influence of external sources. For example, a certain area of the rock is overheated. It starts to char. Charcoal reacts with oxygen. A flammable mixture appears above the surface.
High temperature ignites the formation. Smoldering charcoal appears on the fibers of the boards. To avoid spontaneous combustion, especially if the rock is located near fire sources, you need to remove uneven areas. Then the emergency will not happen.
Combustion products
In the process, gaseous particles and solid particles turn into smoke. Their composition depends on the type of wood. These are compounds of chemical elements with oxygen.
Combustion products are:
- carbon dioxide;
- water vapor;
- nitrogen;
- carbon monoxide;
- sulphur dioxide.
The listed products cannot burn in the future. Except for one exception. It is carbon monoxide. Particulate matter in smoke is soot. The composition of the products depends on the combustion conditions. It may or may not be complete.
If there is not enough air, acrid smoke appears. It is very dangerous for humans. Often inhaling it in large quantities is fatal.
Coal: varieties and characteristics
Coals primarily differ in origin. Charcoal, which is obtained by burning wood, as well as fossil fuels are used as an energy source.
Fossil coals are fuels created by nature. They consist of the remains of ancient plants and bitumen masses, which underwent a number of transformations in the process of sinking underground to great depths. The transformation of starting substances into effective fuel took place at high temperatures and in conditions of oxygen deficiency under the earth. Fossil fuels include brown and hard coals, as well as anthracite.
Brown coals
Among fossil coals, the youngest are brown coals. The fuel got its name from its brown color. This type of fuel is characterized by a large amount of volatile impurities and high moisture content - up to 40%. In this case, the amount of pure carbon can reach 70%.
Due to high humidity, brown coal has a low combustion temperature and low heat transfer. Fuel ignites at 250°C, and the combustion temperature of brown coal reaches 1900°C. The calorific value is approximately 3600 kcal/kg.
As an energy carrier, brown coal in its natural form is inferior to firewood, so it is rarely used for stoves and solid fuel units in private homes. But briquetted fuel is in steady demand.
Brown coals
Brown coal in briquettes is a fuel that has undergone special preparation. By reducing humidity, its energy efficiency increases. The heat transfer of briquetted fuel reaches 5000 kcal/kg.
Stone coals
Hard coals are older than brown coals; their deposits are located at a depth of up to 3 km. In this type of fuel, the content of pure carbon can reach 95%, and volatile impurities - up to 30%. This energy carrier contains no more than 12% moisture, which has a positive effect on the thermal efficiency of the mineral.
The combustion temperature of coal under ideal conditions reaches 2100°C, but in a heating furnace the fuel is burned at a maximum of 1000°C. The heat transfer of coal fuel is 7000 kcal/kg. It is more difficult to ignite - it requires heating up to 400°C to ignite.
Coal energy is most often used to heat residential buildings and other buildings.
Stone wall
Anthracite
The oldest solid fossil fuel, which contains practically no moisture and volatile impurities. The carbon content in anthracite exceeds 95%.
The specific heat transfer of the fuel reaches 8500 kcal/kg - this is the highest indicator among coals. Under ideal conditions, anthracite burns at 2250°C. It ignites at a temperature of at least 600°C - this is an indicator for the lowest calorie types. Ignition requires the use of wood to create the necessary heat.
Characteristics of anthracite
Anthracite is primarily an industrial fuel. Its use in a furnace or boiler is irrational and expensive. In addition to high heat transfer, the advantages of anthracite include low ash content and low smoke.
Ignition temperature and other parameters
The process of coal combustion is a chemical reaction of carbon oxidation that occurs at a high initial temperature with intense heat release. Now it’s simpler: coal fuel cannot ignite like paper; ignition requires preheating to 370-700 ° C, depending on the type of fuel.
If you limit the amount of incoming oxygen (cover the ashpit, switch the TT boiler to smoldering mode), instead of CO2, flammable carbon dioxide CO is formed, which is emitted into the chimney, and the combustion efficiency will significantly decrease. To achieve high efficiency, you need to provide favorable conditions:
Brown coals ignite at a temperature of +370 °C, hard coals - 470 °C, anthracite - 700 degrees. Pre-heating of the heating unit using firewood (sawdust briquettes) is required. Air is supplied to the firebox in excess, the safety factor is 1.3-1.5. Combustion is maintained due to the high temperature of the hot layer of coals lying on the grate
It is important to ensure the passage of oxygen through the entire thickness of the fuel, since air moves through the ash pan due to natural chimney draft. The theoretical combustion temperature and specific heat transfer of various types of fuel are shown in the comparison table
It is noticeable that under ideal conditions any fuel will release maximum heat when interacting with the required volume of air
The theoretical combustion temperature and specific heat transfer of various types of fuel are shown in the comparison table. It is noticeable that under ideal conditions any fuel will release maximum heat when interacting with the required volume of air.
In practice, it is unrealistic to create such conditions, so air is supplied with some excess. The actual combustion temperature of brown coals in a conventional TT boiler lies in the range of 700...800 °C, of rocks and anthracites - 800...1100 degrees.
If you overdo it with the amount of oxygen, energy will begin to be spent on heating the air and simply fly out into the chimney, the efficiency of the furnace will noticeably drop. Moreover, the fire temperature can reach 1500 °C. The process resembles a regular fire - the flame is large, there is little heat. An example of efficient combustion of coal with a retort burner on an automatic boiler is presented in the video:
Complete combustion of coal fuel requires a special approach to the issue. The task is to achieve maximum efficiency of the heat source, not to overheat the coolant and not start a fire due to too high a temperature.
Anthracite is the highest calorific coking coal
You need to get used to each type of coal. It is better to add unfamiliar fuel in small portions, adjusting the draft with a damper and watching the temperature rise. When you have calculated all the combustion nuances of a given brand, fill the firebox 2/3 full.
The combustion temperature of coal is considered the main criterion that allows you to avoid mistakes when choosing fuel. The performance of the boiler and its quality work directly depend on this value.
Coal combustion - What is the formula for coal combustion? — 22 answers
Coal burning
In the Other Education section, answer the question What is the formula for burning coal? given by the author Maria Nasonova, the best answer is Coal + oxygen and fire = Ayyyyyyy hot!!!
Hello! Here is a selection of topics with answers to your question: What is the formula for burning coal?
Reply from CoBRA79922C+O2—>2CO just like that!!
Answer from Irina Zarechkova Finding out the chemical formula of coal is the same as figuring out the chemical formula of borscht. Coal (coals, they are very different and have different compositions) is a mixture of different chemicals, mainly high molecular weight polycyclic aromatic compounds (arenes) with a high carbon content. Coal is not pure carbon with a crystalline lattice, as many people believe. The most obvious way to think of coal is as solidified oil. After all, oil is also a mixture of hydrocarbons, even with a higher carbon content in relation to coal, but no one claims that oil is liquid carbon in its pure form. Thus, if you are interested in the composition of a specific grade of coal, then look for information on arenes (anthracene C14H10 is one of the largest molecules, consisting of three benzene rings, a large amount of carbon in it is noticeable even from the simplified formula; naphthalene C10H8 - two benzene rings; benzene C6H6 - one benzene ring; as well as their modifications and other variants). In addition to polycyclic hydrocarbons, coals contain varying amounts of water and mineral impurities. Based on hydrocarbon content, coals are divided into brown (65-70% carbon, up to 50% volatiles and about 43% water), hard coal (about 80% carbon, up to 32% volatiles and up to 12% water), anthracite (up to 96% water). carbon, less than 8% volatiles). Anthracite - this ancient, shiny and dense coal, which even gives its name to noble black shades of paint, is already similar to what coal is generally considered to be: pure carbon, well, slightly contaminated with impurities. Anthracites are formed at elevated pressure and temperature at greater depths, therefore their composition is closest to graphite, which is precisely an allotropic modification of carbon in its pure form (with a crystal lattice) and can also be considered coal.
Hello! Here are more topics with the answers you need:
Anthracite
Anthracite is the oldest form of fossil coal. It is characterized by a dark black color and has a characteristic metallic sheen. This is the best coal in terms of the amount of heat it releases during combustion.
The amount of moisture and volatile substances in it is very small. About 5-7% for each indicator. And the elemental composition is characterized by an extremely high carbon content:
- Carbon over 90%.
- Hydrogen 1-3%.
- Oxygen 1-1.5%.
- Nitrogen 1-1.5%.
- Sulfur up to 0.8%.
More coal is contained only in graphite, which is a further stage of anthracite coalification.
History of the formation of coal and its types
The entire process of coal formation can be divided into two main stages: the formation of peat and the actual process of coalification - the conversion of peat into coal.
Peat formed on vast water-covered areas from plant remains of varying degrees of decomposition. Some plants rotted completely to a gel-like state, while others retained their cellular structure. Their remains accumulated at the bottom of reservoirs, which gradually turned into swamps. A prerequisite for the formation of peat is the absence of oxygen. There was little oxygen under the water column; during the decomposition of the residues, hydrogen sulfide, methane and carbon dioxide were released, which contributed to the hardening of the residues. Peat formed.
History of coal. It all started many millions of years ago
But not all peatlands were converted to coal. The carbonization process requires: high pressure, high temperature and a long period of time. Depending on the presence of these conditions, the formation of coal occurred or not. First, the peat was carried over by sedimentary rocks, which increased the pressure and temperature inside the peat layer. Under such conditions, brown coal was formed - the first stage of coalification. In some areas, strata displacement occurred, causing brown coal seams to sink (some of the discovered deposits are at depths of more than 6,000 meters). In some places, these processes were accompanied by the rise of magma and volcanic eruptions. High pressure, lack of oxygen and high temperatures contributed to the fact that there was less and less moisture and natural gases in brown coal, and more and more carbon. As water and gases were displaced, brown coal turned into bituminous coal, then, in the presence of high temperatures, into anthracite. The main difference between brown coal and hard coal: brown coal contains more moisture and natural gases and less carbon, which affects the amount of heat released during combustion.
The age of coal is determined by the vegetation remains it contains. Sometimes the prints are very clear
Today, the age of coal deposits is determined by plant remains. The oldest ones date back to the Carboniferous period (345-280 million years ago). During this period, most of the coal basins of North America (eastern and central USA), central and western Europe, southern Africa, China, and India were formed. In Eurasia, most of the coal deposits were formed in the Permian period, some of the small coal basins in Europe date back to the Triassic period. The activity of coal formation increases towards the end of the Jurassic and in the Cretaceous. Around this time, deposits were formed in eastern Europe, the American Rocky Mountains, Indochina and central Asia. Later, mainly brown coals and peat deposits were formed.
Types of coal
Coal is classified according to its moisture, natural gas and carbon content. As the amount of carbon increases, its calorific value increases. The less moisture and volatile substances (gases), the better it tolerates storage and transportation.
Lignite is coal at the first stage of coalification. It differs from brown coal in the smaller amount of water (45%) in its composition and greater heat generation. The structure is fibrous, the color is from brown to black (higher quality). Most often used in the energy sector (at thermal power plants), it is rarely used for heating private houses, as it is poorly stored and has a low calorific value in conventional stoves.
Coal. Lignite. Has a loose layered structure
Subbitominous coal is black in color, has a less pronounced fibrous structure, has a higher calorific value compared to lignite, and has a lower moisture content (30%). It crumbles during transportation and dissipates in the open air. When burned, it emits 5-6 kW/kg. It is used both in the energy sector and in housing and communal services for heating.
Bituminous coal has the highest calorific value and does not lose its qualities during transportation and storage. When burning, it releases 7-9 kW/kg of heat. Some of its types are used for coking.
Anthracite is pitch-black coal. It has the highest hydrocarbon content. It is difficult to light, but it burns for a long time and without soot, and produces a large amount of heat (more than 9 kW/kg). It is anthracite that is most often used for heating.
Anthracite. It has a deep black color and a shiny surface.
Story
Carbon in the form of charcoal was used in ancient times to smelt metals.
Allotropic modifications of carbon—diamond and graphite—have long been known. At the turn of the XVII-XVIII centuries. The phlogiston theory arose, put forward by Johann Becher and Georg Stahl. This theory recognized the presence in each combustible body of a special elementary substance - a weightless fluid - phlogiston, which evaporates during the combustion process. Since when a large amount of coal is burned, only a little ash remains, phlogistics believed that coal was almost pure phlogiston. This is what explained, in particular, the “phlogisticating” effect of coal—its ability to restore metals from “limes” and ores. The later phlogistics, Reaumur, Bergman and others, had already begun to understand that coal was an elemental substance. However, “clean coal” was first recognized as such by Antoine Lavoisier, who studied the process of combustion of coal and other substances in air and oxygen. In the book “Method of Chemical Nomenclature” (1787) by Guiton de Morveau, Lavoisier, Berthollet and Fourcroix, the name “carbon” (carbone) appeared instead of the French “pure coal” (charbone pur). Under the same name, carbon appears in the “Table of Simple Bodies” in Lavoisier’s “Elementary Textbook of Chemistry.”
In 1791, the English chemist Tennant was the first to obtain free carbon; he passed phosphorus vapor over calcined chalk, resulting in the formation of calcium phosphate and carbon. It has been known for a long time that diamond burns without leaving a residue when heated strongly. Back in 1751, the German Emperor Franz I agreed to provide diamond and ruby for combustion experiments, after which these experiments even became fashionable. It turned out that only diamond burns, and ruby (aluminum oxide with an admixture of chromium) can withstand prolonged heating at the focus of the ignition lens without damage. Lavoisier conducted a new experiment on burning diamond using a large incendiary machine and came to the conclusion that diamond is crystalline carbon. The second allotrope of carbon - graphite - in the alchemical period was considered a modified lead luster and was called plumbago; It was only in 1740 that Pott discovered the absence of any lead impurity in graphite. Scheele studied graphite (1779) and, being a phlogistician, considered it a special kind of sulfur body, a special mineral coal containing bound “aerial acid” (CO2) and a large amount of phlogiston.
Twenty years later, Guiton de Morveau turned the diamond into graphite and then into carbonic acid by careful heating.
origin of name
In the 17th-19th centuries, the term “carbon solution” was sometimes used in Russian chemical and specialized literature (Schlatter, 1763; Scherer, 1807; Severgin, 1815); Since 1824, Solovyov introduced the name “carbon”. Carbon compounds have the carbo(n)
- from lat.
carbō (gen. carbōnis
) “coal”.
Conclusions and useful video on the topic
About the calorific value of different types of wood. Comparison of indicators per m3 and kg.
TCT is the most important thermal and operational characteristic of a fuel. This indicator is used in various areas of human activity: heat engines, power plants, industry, home heating and cooking.
Calorific values help to compare different types of fuel according to the degree of energy released, calculate the required mass of fuel, and save on costs.
Do you have anything to add or have questions about the calorific value of different types of fuel? You can leave comments on the publication and participate in discussions - the contact form is in the lower block.