Infrared rays are electromagnetic waves in the invisible region of the electromagnetic spectrum, which begins behind visible red light and ends before microwave radiation between frequencies 1012 and 5∙1014 Hz (or in the wavelength range 1–750 nm). The name comes from the Latin word infra and means "below red".
The uses of infrared rays are varied. They are used for imaging objects in darkness or smoke, heating saunas and heating aircraft wings for de-icing, short-range communications and spectroscopic analysis of organic compounds.
IR Radiation and Heat
Infrared radiation is often called thermal radiation. It should be noted, however, that this is only a consequence of it. Heat is a measure of the translational energy (energy of motion) of atoms and molecules of a substance. "Temperature" sensors don't actually measure heat, but only differences in the IR emissions of different objects.
Many physics teachers traditionally attribute all the thermal radiation of the Sun to infrared rays. But it is not so. Visible sunlight supplies 50% of all heat, and electromagnetic waves of any frequency with sufficient intensity can cause heating. However, it is fair to say that at room temperature, objects produce heat primarily in the mid-infrared region.
IR radiation is absorbed and emitted by the rotations and vibrations of chemically bonded atoms or groups of atoms and, therefore, by many types of materials. For example, window glass that is transparent to visible light absorbs IR radiation. Infrared rays are largely absorbed by water and the atmosphere. Although they are invisible to the eye, they can be felt on the skin.
Characteristics of ionizing radiation. Their impact on humans
Ionizing radiation is any radiation whose interaction with a medium leads to the formation of charges of different signs. Radioactive radiation, high-energy radiation, and X-rays have properties of ionization of the environment. Radioactive radiation is formed as a result of the spontaneous decay of atomic nuclei of elements.
Ionizing radiation includes:
1) gamma radiation - electromagnetic photon radiation emitted during nuclear transformations;
2) characteristic - photon radiation emitted when the energy state of the atom changes;
3) bremsstrahlung - photon radiation emitted when the kinetic energy of charged particles changes;
4) X-ray – a combination of bremsstrahlung and characteristic radiation,
5) corpuscular - radiation consisting of particles with a rest mass different from zero, alpha and beta particles, protons, neutrons, etc.
The main regulatory documents regulating the safety of working with sources of ionizing radiation are “Radiation Safety Standards (NRB-2000)” and “Basic Sanitary Rules for Ensuring Radiation Safety (OSP-2002)”.
In accordance with NRB-2000, three categories of exposure are established: Category A – professional exposure of persons working directly with sources of ionizing radiation. Category B – exposure of persons working in adjacent rooms, but not directly engaged in work associated with radiation hazard. Category B – exposure of the population of all age categories.
Ionizing radiation includes X-rays, alpha, beta, gamma radiation, etc.
Alpha radiation is a stream of nuclei of helium atoms. The penetrating power of alpha particles, i.e. the ability to pass through a layer of any substance of a certain thickness is small. Therefore, the external impact of alpha particles on a living organism is not dangerous. However, alpha particles are highly ionizing, and their entry into the body through the respiratory tract, gastrointestinal tract, or wounds causes serious illness.
Beta radiation consists of a stream of electrons. They have significantly greater penetrating, but lower ionizing ability compared to alpha particles. It is the high penetrating ability of electrons that is a dangerous factor when irradiated by these particles.
Gamma rays are electromagnetic radiation with a very short wavelength. They not only penetrate deeply into the body, but also have a strong ionizing effect. As a result, gamma radiation is extremely dangerous for humans.
Ionization of body tissues leads to their destruction due to the splitting of water (its content in living tissue is 72%) and the entry of the resulting substances into a chemical reaction with protein compounds.
Radiation can cause hair loss, brittle nails, disruption of the gastrointestinal tract, cataracts, changes in hereditary functions, acute or chronic radiation sickness.
Throughout life, a person is exposed to radioactive radiation emanating from soil and structures, but it, as a rule, does not cause significant changes in the body.
Earth as a source of infrared radiation
The surface of our planet and clouds absorb solar energy, most of which is released into the atmosphere in the form of infrared radiation. Certain substances in it, mainly steam and water droplets, as well as methane, carbon dioxide, nitrogen oxide, chlorofluorocarbons and sulfur hexafluoride, absorb in the infrared region of the spectrum and re-emit in all directions, including to Earth. Therefore, due to the greenhouse effect, the earth's atmosphere and surface are much warmer than if there were no substances that absorb infrared rays in the air.
This radiation plays an important role in heat transfer and is an integral part of the so-called greenhouse effect. On a global scale, the influence of infrared rays extends to the Earth's radiation balance and affects almost all biosphere activity. Almost every object on the surface of our planet emits electromagnetic radiation mainly in this part of the spectrum.
IR Regions
The infrared range is often divided into narrower sections of the spectrum. The German standards institute DIN has defined the following wavelength ranges of infrared rays:
- near (0.75-1.4 µm), commonly used in fiber optic communications;
- short-wave (1.4-3 microns), starting from which the absorption of IR radiation by water increases significantly;
- medium wave, also called intermediate (3-8 microns);
- long-wave (8-15 microns);
- long-range (15-1000 µm).
However, this classification scheme is not universally used. For example, some studies report the following ranges: near (0.75-5 µm), medium (5-30 µm) and long (30-1000 µm). Wavelengths used in telecommunications are classified into separate bands due to limitations of detectors, amplifiers, and sources.
The general notation system is justified by human reactions to infrared rays. The near-infrared region is closest to the wavelength visible to the human eye. Mid- and far-IR radiation gradually moves away from the visible part of the spectrum. Other definitions follow different physical mechanisms (such as emission peaks and water absorption), and the newest ones are based on the sensitivity of the detectors used. For example, conventional silicon sensors are sensitive in the region of about 1050 nm, and indium gallium arsenide is sensitive in the range from 950 nm to 1700 and 2200 nm.
There is no clear boundary between infrared and visible light. The human eye is much less sensitive to red light above 700 nm, but intense light (from the laser) can be seen down to about 780 nm. The beginning of the infrared range is defined differently in different standards - somewhere between these values. Typically this is 750 nm. Therefore, visible infrared rays are possible in the range of 750–780 nm.
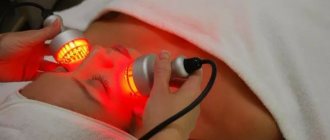
Treatment methods
Therapy is of two types, namely general and local. As for local effects, treatment is carried out on a specific part of the patient’s body. During general therapy, the use of light therapy is aimed at the entire body.
The procedure is carried out twice a day, the session duration ranges from 15-30 minutes. The general treatment course contains at least five to twenty procedures. Make sure you have infrared protection for your face ready. Special glasses, cotton wool or cardboard covers are used for the eyes. After the session, the skin becomes covered with erythema, namely redness with blurred boundaries. The erythema disappears an hour after the procedure.
Symbols in communication systems
Near-infrared optical communications are technically divided into a number of frequency bands. This is due to various light sources, absorbing and transmitting materials (fibers) and detectors. These include:
- O-band 1,260-1,360 nm.
- E-band 1,360-1,460 nm.
- S-band 1,460-1,530 nm.
- C-band 1,530-1,565 nm.
- L-band 1.565-1.625 nm.
- U-band 1.625-1.675 nm.
Thermography
Thermography, or thermal imaging, is a type of infrared image of objects. Since all bodies emit infrared radiation, and the intensity of radiation increases with temperature, specialized cameras with infrared sensors can be used to detect it and take pictures. In the case of very hot objects in the near-infrared or visible region, this method is called pyrometry.
Thermography is independent of visible light illumination. Therefore, it is possible to “see” the environment even in the dark. In particular, warm objects, including people and warm-blooded animals, stand out well against a cooler background. Infrared landscape photography enhances the display of objects based on their heat output, making blue skies and water appear almost black, while green foliage and skin are brought out vividly.
Historically, thermography has been widely used by military and security services. In addition, it has many other uses. For example, firefighters use it to see through smoke, find people, and locate hot spots during a fire. Thermography can reveal abnormal tissue growth and defects in electronic systems and circuits due to their increased heat generation. Electricians who maintain power lines can detect overheating connections and parts that indicate a problem and eliminate the potential hazard. When insulation fails, building professionals can see heat leaks and improve the efficiency of cooling or heating systems. In some high-end cars, thermal imagers are installed to assist the driver. Thermographic imaging can monitor several physiological reactions in humans and warm-blooded animals.
The appearance and method of operation of a modern thermographic camera are no different from those of a conventional video camera. The ability to see in the infrared spectrum is such a useful feature that the ability to record images is often optional and a recording module is not always available.
Types of Heat Transfer
There are three main types of heat transfer: conduction , convection , and thermal radiation .
Thermal conduction is the direct transfer of heat between two physical bodies. The symbol "k" is a measurement of how well various substances transfer heat. The amount of heat that can be transferred through a surface depends on the temperature difference, the surface area, the thermal conductivity of the material, and the thickness of the material.
Convection is a type of heat exchange (heat transfer) in which internal energy is transferred due to the movement of matter (liquids or gases). When a substance is heated, it expands and its density increases. Heat flow occurs when warm substances rise and cold substances sink. Thermal energy (heat) is transferred by particles in streams moving from one place to another. Convection can be natural, when nearby substances (liquids or gases) are used, or forced, when a pump or fan is used.
Convection also depends on surface area. If the surface area in contact with the substance increases, then the range of heat transfer also increases. That's why you can most often see ribbed convection devices for more efficient operation.
Radiation is a non-contact heat transfer that does not require any medium. Thermal radiation is electromagnetic radiation emitted by bodies due to their internal energy. The higher the body temperature, the stronger its thermal radiation. Heat transfer occurs when the transferred energy collides with a body and is absorbed by it.
Other images
In IR photography, the near-infrared region is captured using special filters. Digital cameras tend to block IR radiation. However, cheap cameras that do not have appropriate filters can “see” in the near-infrared range. In this case, usually invisible light appears bright white. This is especially noticeable when shooting near illuminated infrared objects (for example, a lamp), where the resulting interference makes the image faded.
Also worth mentioning is T-beam imaging, which is imaging in the far terahertz range. The lack of bright sources makes such images technically more challenging than most other IR imaging techniques.
LEDs and lasers
Artificial sources of infrared radiation include, in addition to hot objects, LEDs and lasers. The former are small, inexpensive optoelectronic devices made from semiconductor materials such as gallium arsenide. They are used as opto-isolators and as light sources in some fiber optic communication systems. High-power optically pumped IR lasers operate on the basis of carbon dioxide and carbon monoxide. They are used to initiate and modify chemical reactions and separate isotopes. In addition, they are used in lidar systems for determining the distance to an object. Infrared radiation sources are also used in rangefinders of automatic self-focusing cameras, security alarms and optical night vision devices.
Receivers
Spitzer Infrared Space Telescope
Main mirror with a diameter of 85 cm
made of beryllium and cooled to a temperature of 5.5
K
to reduce the mirror's own infrared radiation.
The telescope was launched in August 2003 under NASA's Four Great Observatories
, including:
- Compton Gamma-Ray Observatory (1991–2000, 20 keV
–30
GeV
), see 100 MeV Gamma Ray Sky, - X-ray Observatory "Chandra" (1999, 100 eV
-10
keV
), - Hubble Space Telescope (1990, 100–2100 nm
), - Spitzer infrared telescope (2003, 3–180 µm
).
The Spitzer telescope is expected to have a lifespan of about 5 years. The telescope received its name in honor of astrophysicist Lyman Spitzer (1914–97), who in 1946, long before the launch of the first satellite, published the article “Advantages for Astronomy of an Extraterrestrial Observatory,” and 30 years later convinced NASA and the American Congress to begin developing a space telescope. Hubble."
Connection
Infrared wavelengths are used to transmit data over short distances, such as between computer peripherals and personal digital assistants. These devices typically comply with IrDA standards.
IR communications are typically used indoors in areas with high population density. This is the most common way to remotely control devices. The properties of infrared rays do not allow them to penetrate walls, and therefore they do not interact with equipment in adjacent rooms. In addition, IR lasers are used as light sources in fiber optic communication systems.
Conclusion
Infrared heating is a new product on the modern market with great potential. Despite the fact that this technology was invented at the beginning of the last century, it is only now that it is being actively used for heating rooms. In the construction of large facilities, such heating is simply irreplaceable, because it significantly saves energy, is convenient to install and use, and has a number of other advantages. It is safe to say that this is the technology of the future, since in the context of the desire to reduce energy consumption, infrared heating is a big and bold step towards the goal.
Request a call
On-line calculator
Spectroscopy
Infrared radiation spectroscopy is a technology used to determine the structures and compositions of (mainly) organic compounds by studying the transmission of infrared radiation through samples. It is based on the properties of substances to absorb certain frequencies, which depend on the stretching and bending inside the molecules of the sample.
The infrared absorption and emission characteristics of molecules and materials provide important information about the size, shape, and chemical bonding of molecules, atoms, and ions in solids. The energies of rotation and vibration are quantized in all systems. IR radiation of energy hν emitted or absorbed by a given molecule or substance is a measure of the difference between certain internal energy states. They, in turn, are determined by atomic weight and molecular bonds. For this reason, infrared spectroscopy is a powerful tool for determining the internal structure of molecules and substances or, when such information is already known and tabulated, their quantities. IR spectroscopy techniques are often used to determine the composition and therefore the origin and age of archaeological samples, as well as to detect forgeries of works of art and other objects that, when examined under visible light, resemble the originals.
The benefits and harms of infrared rays
Long-wave infrared radiation is used in medicine for the following purposes:
- normalization of blood pressure by stimulating blood circulation;
- cleansing the body of heavy metal salts and toxins;
- improves blood circulation in the brain and memory;
- normalization of hormonal levels;
- maintaining water-salt balance;
- limiting the spread of fungi and microbes;
- pain relief;
- relieving inflammation;
- strengthening the immune system.
At the same time, IR radiation can cause harm in acute purulent diseases, bleeding, acute inflammation, blood diseases, and malignant tumors. Uncontrolled prolonged exposure leads to skin redness, burns, dermatitis, and heat stroke. Short-wave infrared rays are dangerous to the eyes - photophobia, cataracts, and visual impairment may develop. Therefore, only long-wave radiation sources should be used for heating.
First aid for heatstroke
If complications cannot be avoided, it is necessary to take a set of certain measures.
When providing first aid for heatstroke, the following steps should be taken.
- Call an ambulance.
- Move the victim to a cool place, preferably in the shade, where there is access to fresh air.
- Make it easier for him to breathe by removing or unbuttoning his clothes. Give validol.
- Place the victim in a horizontal position, raising his legs.
- Give the victim 1 liter of water with a little salt.
- Cool the person by wrapping him in a cold wet towel and applying ice to his forehead.
- In case of loss of consciousness, it is necessary to give the victim a sniff of ammonia.