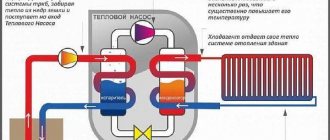
Thermal energy has several measurement options.
Energy power, which is measured in Watts (W, mW and kW), is most often indicated on heating boilers, heaters, etc.
Another unit of energy measurement, the gigocalorie (Gcal), may be encountered when installing heat meters.
Also, the supplied heat is sometimes indicated in Gcal on payment receipts.
And if the calculation is accepted by the management company in one unit, and the meter shows another, it may be necessary to monthly convert Gcal to kW and back. Once you understand everything once, you can learn how to do it quickly and easily.
What are calories
During the construction of buildings, all measurements and thermal calculations are made in gigacalories. Utilities also prefer this unit of measurement for its proximity to real life and the possibility of calculations on an industrial scale.
We remember from the school course that a calorie is the work needed to heat 1 gram of water per unit °C (at a certain atmospheric pressure).
In life you have to deal with Kcal and Gcal, gigacalories.
- 1 Kcal = 1 thousand cal.
- 1 Gcal = 1 million Kcal, or 1 Billion. cal.
The following measurement can be used in heating receipts:
- Gcal;
- Gcal/hour.
In the first case, we mean the supplied heat for a certain period (this could be a month, a year or a day). Gcal/hour is a characteristic of the power of a device or process (such a unit of measurement can indicate the performance of a heating device or the rate of heat loss of a building in winter). The receipts imply heat that was released in 1 hour. Then, to recalculate for a day, you need to multiply the number by 24, and for a month by another 30/31.
1 Gcal/hour = 40 m3 of water, which was heated to 25 °C in 1 hour.
Also, a gigacalorie can be tied to the volume of fuel (solid or liquid) Gcal/m3. And it shows how much heat can be obtained from a cubic meter of this fuel.
What is a calorie?
The calorie is not part of the international metric measurement system, but the concept is widely used to denote the amount of energy released. It indicates how much energy must be expended to heat 1 g of water so that this volume increases the temperature by 1 °C under standard conditions.
There are 3 generally accepted designations, each of which is used depending on the area:
- The international value of a calorie, which is equal to 4.1868 J (Joule), and is designated as “cal” in the Russian Federation and cal in the world;
- In thermochemistry - a relative value approximately equal to 4.1840 J with the Russian designation calth and the worldwide designation calth;
- A 15-degree calorie value equal to approximately 4.1855 J, which is known in Russia as “cal15”, and in the world as cal15.
Initially, the calorie was used to find the amount of heat released during the generation of fuel energy. Subsequently, this value began to be used to calculate the amount of energy expended by an athlete when performing any physical activity, since the same physical laws apply to these actions.
Since fuel is needed to generate heat, then, by analogy with heat energy in simple life, to produce energy the body also needs “refueling” - food that people take regularly.
A person receives a certain amount of calories, depending on what product he consumes.
The more calories a person receives from food, the more energy he receives for exercise. However, people do not always consume the amount of calories that is necessary to maintain normal vital processes of the body and perform physical activity. As a result, some people lose weight (with a calorie deficit), while others gain weight.
Calorie content is the amount of energy received by a person as a result of the absorption of a particular product.
Based on this theory, many principles of diets and rules of healthy eating are built. The optimal amount of energy and macronutrients that a person needs per day can be calculated in accordance with the formulas of famous nutritionists (Harris-Benedict, Mifflin-St. Geor), using standard parameters:
- Age;
- Height;
- Weight;
- Example of daily activity;
- Lifestyle.
These data can be used by changing them to suit you - for painless weight loss it is enough to create a deficit of 15-20% of daily calories, and for healthy weight gain - a similar surplus.
How to convert energy units?
On the Internet you can actually find a huge number of online calculators that convert the required values automatically.
When it comes to figuring it all out, there are often lengthy formulas and proportions that can be off-putting to the average consumer who graduated from school many years ago.
But it’s possible to figure everything out! You will need to remember 1 or 2 numbers, an action, and you can easily make the translation offline, on your own.
How to convert kW to Gcal/h
Key indicator for converting data from kilowatts to calories:
1 kW = 0.00086 Gcal/hour
To find out how many Gcal is obtained, you need to multiply the available number of kW by a constant value, 0.00086.
Let's look at an example. Suppose you need to convert 250 kW into calories.
250 kW x 0.00086 = 0.215 Gcal/hour.
(More accurate online calculators will show 0.214961).
To ensure 100% reliability of the heating system, a good solution would be to install an electric boiler along with a solid fuel boiler. It is quite possible to assemble such a structure with your own hands and thereby save money.
Instructions for assembling an electrolyzer with your own hands are presented here.
The heating season has arrived, but the radiators are still cold? Don’t look for ways to warm yourself, demand respect for your rights. Follow the link for information on where to call and what to do if there is no heating.
How are Gcal calculated for hot water and heating?
Heating is calculated using formulas similar to the formulas for finding the value of Gcal/h.
An approximate formula for calculating payment for warm water in residential premises:
P i gv = Vi gv * T x gv + (V v cr * Vi gv / ∑ Vi gv * T v cr)
Values used:
- P i gv – the required value;
- V i gv – volume of hot water consumption for a certain time period;
- T x gw – established tariff fee for hot water supply;
- V v gv – the volume of energy expended by the company that heats it and supplies it to residential/non-residential premises;
- ∑ V i gw – the sum of warm water consumption in all rooms of the house in which the calculation is made;
- T v gv – tariff payment for thermal energy.
This formula does not take into account the atmospheric pressure indicator, since it does not significantly affect the final desired value.
The formula is approximate and is not suitable for independent calculation without prior consultation. Before using it, you need to contact local utility services for clarification and adjustment - perhaps they use other parameters and formulas for calculation.
Calculating the size of the heating fee is very important, since often impressive amounts are not justified
The result of the calculations depends not only on the relative temperature values - it is directly influenced by the tariffs established by the government for the consumption of hot water supply and space heating.
The computational process is greatly simplified if you install a heating meter in an apartment, entrance or residential building.
It is worth considering that even the most accurate meters can allow errors in calculations. It can also be determined by the formula:
E = 100 *((V1 – V2)/(V1 + V2))
The presented formula uses the following indicators:
- E – error;
- V1 – volume of hot water supply consumed upon receipt;
- V2 – consumed hot water at the outlet;
- 100 is an auxiliary coefficient that converts the result into percentages.
In accordance with the requirements, the average error of the calculation device is about 1%, and the maximum permissible is 2%.
Video: example of calculating heating fees
Convert Gcal to kW/h
The opposite situation is when you need to convert Gcal to kW. You need to know how many kW contains 1 Gcal
1 Gcal = 1163 kW.
This means that one gigacalorie of heat will need to be consumed to produce 1163 kilowatts of energy.
Or vice versa: 1163 kW of energy will be required to produce one Gcal of heat.
To convert the number of gigocalories you know into kilowatts, you need to multiply the existing Gcal indicator by 1163.
Example:
It is necessary to convert 0.5 Gcal into kilowatts.
0.5 x 1163 = 581.5 kW.
Calculation examples
Now we come to the most important thing. How to convert one quantity to another using the given ratios? It's not all that complicated. Let's look at this with examples.
Example 1
Thermal power of the boiler is 30 kW. What is its equivalent power, expressed in Gcal/h?
Solution. Since 1 kW= 0.00085984523 Gcal/h, then 30 kW=30* 0.00085984523 Gcal/h=0.0257953569 Gcal/h.
Example 2
It is estimated that an air conditioner with a power of at least 2.5 kW is required to cool an office. An air conditioner with a capacity of 8000 BTU/h was selected for purchase. Is your air conditioner powerful enough to cool your office?
Solution. Since 1 BTU/h=0.2931 W, then 8000 BTU/h=2344.8 W=2.3448 kW. This value is less than the calculated 2.5 kW, so the selected air conditioner is not suitable for installation.
Example 3
The heat supply organization supplied 0.9 Gcal of heat per month. What power do you need to install a radiator so that it produces the same amount of heat per month?
Solution. Let’s assume that heat was supplied to the house evenly over one month (30 days), so the thermal power supplied by the boiler house can be found by dividing the total amount of heat by the number of hours in the month: P = 0.9 Gcal/(30*24 hours) =0.00125 Gcal/h. This power in terms of kilowatts will be equal to P=1163 kW*0.00125=1.45375 kW.
Conversion table
A quick translation of round numbers can be done using tables:
| Gcal to kW | ||||||||
| gigacalories/hour | 1 | 2 | 3 | 4 | 5 | 10 | 15 | 20 |
| kW | 1163 | 2326 | 3489 | 4652 | 5815 | 11630 | 17445 | 23260 |
| kW to Gcal | |||||||
| kW | 1000 | 5000 | 10000 | 30000 | 50000 | 100000 | 500000 |
| gcal/h | 0.85984 | 4.29922 | 8.5984 | 25.795 | 42.992 | 85.984 | 429.9226 |
Gigacalories or kilowatts
Let us finally understand what is the difference between these units of measurement. Let us have a heating device, for example, a kettle. Take 1 liter of cold tap water (temperature t1=15°C) and boil it (heat to temperature t2=100°C). The electric power of the kettle is P=1.5 kW. How much heat will water absorb? To find out, let’s apply the formula we are familiar with, taking into account that the mass of 1 liter of water m=1 kg: Q=4183 [J/(kg*°C)]*1 kg*(100°C-15°C)= 355555 J=84922.8528 cal≈85 kcal.
How long does it take for the kettle to boil? Let all the energy of the electric current go to heating the water. Then we will find the unknown time using the energy balance: “The energy consumed by the kettle is equal to the energy absorbed by water (without taking into account losses).” The energy consumed by the kettle during time τ is equal to P*τ. The energy absorbed by water is equal to Q. Then, based on the balance, we obtain P*τ=Q. Hence the heating time of the kettle will be: τ=Q/P=355555 J/1500 W≈237 s≈4 min. The amount of heat transferred by a kettle to water per unit of time is its thermal power. In our case, it will be the value Q/τ=84922.8528 cal/237 s≈358 cal/s=0.0012888 Gcal/h.
Thus, kW and Gcal/h are units of power , and Gcal and MJ are units of heat and energy. How can such calculations be applied in practice? If we receive a receipt for payment for heating, then we pay for the heat that the supplying organization supplies us through pipes. This heat is taken into account in gigacalories, i.e. in the amount of heat consumed by us during the billing period. Should this unit be converted to joules? Of course not, because we are simply paying for a specific number of gigacalories.
However, it is often necessary to choose certain heating devices for a house or apartment, for example, an air conditioner, radiator, boiler or gas boiler. In this connection, it is necessary to know in advance the thermal power required to heat the room. Knowing this power, you can select the appropriate device. It can be indicated both in kW and in Gcal/h, as well as in BTU/h units (British Thermal Unit, h - hour). The following cheat sheet will help you convert kW to Gcal/h, kW to BTU/h, Gcal to kWh and BTU to kWh.
Memo 2
- one W=one J/s=0.2388459 cal/s=859.8452 cal/h=0.8598 kcal/h;
- one kW=one kJ/s=1000 J/s=238.8459 cal/s=859845.2279 cal/h=0.00085984523 Gcal/h;
- one MW=one MJ/s=one million J/s=1000 kW=238845.8966 cal/s=0.85984523 Gcal/h;
- one Gcal/h=one billion cal/h=1163000 W=1163 kW=1.163 MW=3968156 BTU/h;
- one BTU/h=0.2931 W=0.0700017 cal/s=252.0062 cal/h=0.2520062 kcal/h;
- one W=3.412 BTU/h, one kW=3412 BTU/h, one MW=3412000 BTU/h.
How is BTU/h determined and what is it used for? 1 BTU is the amount of heat required to warm 1 pound of water 1° Fahrenheit (°F). This unit of measurement is used primarily to indicate the heating output of installations such as air conditioners.