Since the main task of heating systems is to transfer heat from heating boilers to radiators, a key factor in the efficiency of such a system is the choice of coolant. As you know, the coolant can be water, oil or an alcohol-containing solution, but the most practical in most respects will be a special non-freezing liquid - antifreeze.
Antifreeze is preferred as a coolant due to its unique properties:
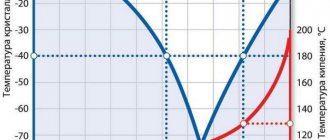
- Low crystallization (freezing) threshold;
- Elevated boiling point from 105°C to 135°C;
- Low viscosity, making heat transfer even more efficient;
- Availability on the market.
Antifreezes are divided into 3 conditional groups: based on glycerin, propylene glycol or ethylene glycol. Each of these substances has its own characteristics and chemical composition, which is why the use of a particular antifreeze depends on many parameters, such as the area of application, the type of equipment, the materials from which it is made, climate and others. Let's take a closer look at them.
How and with what to dilute the coolant?
Climate control systems are an effective, but at the same time quite capricious mechanism that can fail at the most unexpected moment.
Heating equipment - at the height of the winter season, air conditioning systems - in the summer heat. The reasons are varied: from sudden power outages to wear and tear of individual components of the utility network. If you use water as the coolant for the climate system, then at subzero temperatures there is a high risk of the heating circuit freezing. This leads to irreparable consequences - destruction of pipelines and equipment. To avoid costly repairs, it is important to use non-freezing liquids - industrial antifreezes and coolants. Glycol-based compositions are very popular in the industrial coolant market. The consumer has a choice: buy a ready-made composition with a package of anti-corrosion additives or purchase a solution of the glycol concentration of interest in bulk. In order to comply with all requirements, it is important to know the composition of glycol coolants, thermophysical properties, operating features and dilution rules.
201301230950_15
Why is methanol added to the coolant?
Mainly - to reduce the overall viscosity/density of the “mixture” into which the manufacturer swelled diethylene glycol, glycerin, and propylene glycol. But methanol is a low-boiling liquid (boiling point = 64.7°C at atmospheric pressure!), and therefore its presence immediately lowers the overall boiling point of the “mixture,” which is a scourge in the summer. In addition, methanol is toxic, volatile, and a carcinogen. It also increases cavitation wear. Finally, methanol is a strong solvent and, when hot, damages rubber and polymer parts that it comes into contact with.
What do designations like G11, G12 mean?
These designations are not official. The fashion for them was generated by Volkswagen, for whose cars the VW coolant G11 and VW coolant G12 were produced. Thus, the G11 and G12 symbols are associated with this brand - and only with it. As a rule, antifreeze manufacturers do not indicate the components included in the antifreeze. But in any case, it makes no sense to look for G11 or G12 in the store.
Coolant requirements for the climate system
Any coolant is a working medium intended for the redistribution and transfer of thermal energy. A correctly selected and prepared composition allows you to optimize the operation of the circuit, increase the efficiency of the utility network, protect equipment from corrosion and minimize the risk of failure.
When choosing a coolant, it is important to consider the following features:
- The high heat capacity of the composition allows it to accumulate and deliver thermal energy to radiators with minimal losses.
- Wide range of operating temperatures corresponding to the climatic conditions of the region and the operating characteristics of the facility.
- Inertness of antifreeze in relation to pipes, radiators, circulation pump and other system equipment.
- Resistance to the formation of corrosion spots on the washed surface.
- Recommended service life of the composition.
The main advantage of modern glycol-based antifreezes for industrial premises is the ability to withstand negative temperatures, protect equipment from scale and rust formation. This is ensured by the introduction of anti-corrosion additives into the composition, which extend the service life of the coolant. In the case of products from the Hot Stream line from TECHNOFORM, the recommended service life is 5-10 years.
Compared to water, glycol solution:
- Has a viscosity 3-5 times higher. This requires the use of pumping equipment with a productivity of at least 10-15% higher.
- Less heat-intensive. At the same heating, glycol-based coolants accumulate and release approximately 15% less thermal energy.
- It is more fluid, which places increased demands on sealing materials and system connections.
With all the features described above, glycol has an incomparable advantage: even when cooled to extremely low temperatures (-60 degrees), the solution retains its original properties and does not freeze.
Rules for operating complexes with glycerin antifreeze
Glycerin coolants have a long service life if the basic rules are observed:
- Antifreeze must not be allowed to overheat. Otherwise, the anti-corrosion impurities at the heart of its composition may disintegrate and form deposits on the surface of the heating elements, which impairs the operation of the heating system as a whole;
- The low coefficient of surface tension of the composition helps reduce swelling of the seals. To reduce the likelihood of leaks, it is necessary to make additional tightening at the junctions of different elements;
- At low temperatures, the coolant in the pipes will have a viscous state with individual crystals of the substance. When starting the equipment, you must first turn on the minimum speed of the heater and increase it gradually. Such a start will avoid premature breakdown of the boiler. The heated composition will have all its original properties.
We recommend reading: Piping an electric heating boiler, power calculation, principle of operation, device, diagram
How to properly dilute the coolant and is it worth doing?
First, let's answer the main question: if you are interested in the possibility of a process from the point of view of thermophysics, yes, it is possible. Before you begin preparing the solution for pouring into the system, you need to carry out a number of preparatory measures. First of all, calculate the required proportions taking into account the concentration of the original solution and the freezing point of the finished product after dilution. You can mix water and antifreeze in two ways: by pouring separately until the operating pressure in the circuit is reached, or by connecting the components of the solution in advance. The first method is rarely used, because Separate addition of water and glycol is fraught with serious problems:
- Uneven heating in certain sections of the pipeline due to poor mixing of liquids.
- Interruptions in the operation of the pump or its stop caused by heterogeneity of the medium.
- Foaming of the solution, the consequences can only be eliminated by completely draining the antifreeze from the system.
Is it possible to dilute the coolant with water?
It is better to ask this question to the manufacturer. Some working compositions are not recommended to be diluted with water, others are perfectly diluted without loss of thermophysical properties. For example, concentrated solutions of glycols can be purchased in bulk and then independently brought to a given working concentration. The only requirement is to use demineralized water when diluting, because... High salt content may cause sediment to form.
Features of dilution of ethylene glycol
To dilute concentrated ethylene glycol, demineralized softened water with a minimum content of magnesium and calcium salts is used. If anti-corrosion additives are added during the dilution process, the water hardness should not exceed 5 mg per equivalent. For convenience, you can use the following table:
| Freezing point of the working composition | Volume of ethylene glycol, in liters | Volume of water, in liters |
| -20 °C | 54 | 60 |
| -25 °C | 60 | 40 |
| -30 °C | 65 | 35 |
| -40 °C | 77 | 23 |
Important! It is not recommended to use pure ethylene glycol. It has increased viscosity and low heat capacity, which negatively affects the efficiency of the equipment.
Concentration of glycerol by mass and volume in aqueous solution
The table below shows the ratio of the concentration of glycerol in an aqueous solution by weight and volume.
| Glycerol content by weight (percentage) | ||||||||
| 5% | 10% | 20% | 30% | 40% | 50% | 60% | 70% | |
| Glycerin concentration by volume (percentage) | 4,0% | 8,1% | 16,58% | 25,49% | 34,84% | 44,63% | 54,86% | 65,56% |
Rules for diluting propylene glycol
To prepare a coolant based on concentrated propylene glycol, demineralized water is used. Dilution in different proportions, by volume or weight, is acceptable. For convenience, you can use the following information:
| Volume concentration in % | Density at 20°C, g/cm 3 | Freezing temperature, °C |
| 25 | 1,023 | -10 |
| 30 | 1,029 | -13 |
| 35 | 1,033 | -17 |
| 40 | 1,037 | -21 |
| 45 | 1,042 | -26 |
| 50 | 1,045 | -32 |
Propylene glycol coolants are not used in systems with galvanized pipes; prolonged exposure leads to peeling of the material.
Keep in mind that when preparing working trains, precautions and fire safety measures must be observed. Work may only be carried out in a well-ventilated area, wearing personal protective equipment. But it is better to buy a ready-made solution and entrust pouring the liquid into the system to professionals.
Important! Do not mix coolants from different manufacturers. Each brand uses its own package of corrosion inhibitors, which can conflict in composition and lead to loss of properties.
Description of the substance
Glycerol is a trihydric alcohol, which differs from ethanol, which has only one atom. The substance is a clean transparent liquid with high viscosity. Glycerin has no color. Alcohol is a food additive. It acts as a preservative, which makes the products thick enough.
Glycerol is made up of three elements: carbon, hydrogen and oxygen. The substance is often obtained from propylene. During its chlorination at high temperatures (from 450 to 5000C), allyl chloride appears. Hypochlorous acid is added to this compound, resulting in the formation of chlorohydrins. At the last stage, they are saponified with alkali. Eventually they turn into alcohol.
The substance can be either plant or technical. The first option is safe for human health. The use of the second can negatively affect the functioning of internal organs. Industrial alcohol is not used in the food industry or in the manufacture of liquids for electronic cigarettes.
Conclusion
Glycol coolant is more technologically advanced, safe and effective than water. In some operating conditions it is indispensable. On sale you can find both ready-made formulations and solutions of glycols of various concentrations with a package of additives, which theoretically can be adjusted to the required parameters.
Self-mixing of ethylene and propylene glycol with water is carried out before filling the system, taking into account the manufacturer’s recommendations. But it is best to purchase a solution of ethylene or propylene glycol in bulk. A variety of options, high-quality anti-corrosion additives made in Belgium - all this guarantees long-term and productive operation of the system.
Source
What is better to use for a home heating system: water or antifreeze?
The standard coolant for autonomous heating systems of private houses is water. It has been used for many years. But recently, manufacturers have begun to propose switching to antifreeze, noting a number of its advantages. To understand whether it is worth using anti-freeze fluid, whether it is a common marketing ploy, and what is generally better for the installed heating system, you should consider and compare the two types of coolant.
The use of antifreeze in the heating system of a private house
Even if the heating system is highly reliable and of good quality, the possibility of freezing in winter cannot be ruled out. The use of antifreeze in the heating system of a private home significantly reduces the risk of this problem. Let's consider what advantages “anti-freeze” devices have, what requirements they must meet, and what are the rules for their use.
Monoethylene glycol: history of production, properties, area of use
Monoethylene glycol (MEG) is a dihydric alcohol, the simplest representative of the category of polyhydric alcohols, also called polyols. It is a colorless, somewhat oily liquid with a sweetish taste and odorless. Many people ask the question: are there any differences between monoethylene glycol and ethylene glycol? There is no difference. These are names of the same compound.
TD Roka Chemicals LLC is a manufacturer of antifreeze used as a coolant in heating, air conditioning, heating or cooling systems, as well as automotive coolants.
Our managers will not only place your order, but will also help you formulate it correctly, as well as provide all the necessary information and materials for successful implementation.
To order products, you can contact the sales department by phone +7(8313) 27-52-47.
Products are shipped in any container suitable for you and sent in any way convenient for you to the following cities:
- Buryatia, Altai, Karelia, Komi, Sakha (Yakutia), Tyva, Krasnoyarsk, Vladivostok, Khabarovsk, Blagoveshchensk, Irkutsk, Petropavlovsk-Kamchatsky, Murmansk, Perm, Yuzhno-Sakhalinsk, Tomsk, Tyumen, Khanty-Mansiysk. Moscow, St. Petersburg, Kazan, Ekaterinburg, Yakutsk
Source
Sticky type
Glycerol, an odorless and sweet-tasting trihydric alcohol, was first synthesized back in 1779 by Swedish chemist Carl Wilhelm Scheele by heating olive oil with lead oxide. Glycerin became more widespread after the mid-19th century, when its industrial production began. Initially, the key area of its application was pharmacology, but by the beginning of the 20th century, with the development of engineering systems and mechanical engineering, it became the basis of a coolant.
A hundred years ago, glycerin was mixed with water, which allowed the working fluid to withstand temperatures up to 40 degrees below zero without freezing and heating up to 250 degrees without boiling. But even then, consumers faced serious problems when using such a mixture - it was not fluid enough to provide stable and uniform cooling. In addition, only very powerful pumps could pump glycerin diluted with water through the system. During the experiments, a new antifreeze composition was found, in which ethanol or methanol acted as a glycerin solvent, but it did not solve the critical problems when using such mixtures.
Glycerin based coolant
A glycerin-based coolant is a solution of glycerin in water with the addition of various additives and dye.
The presence of glycerin in the coolant reduces its freezing point, which makes the heating system (CO) more resistant to malfunctions that lead to temporary cessation of operation of the heating boiler.
The probability that the glycerin-based coolant will freeze in the lines, which will lead to their rupture and failure of the CO, is significantly less than that which exists for systems using only water as a coolant.
Glycerin in the heating system is the main factor influencing the further choice of the CO project for a private house, the type of heating boiler, the power of installed heating devices (convectors or radiators), the power of the main pump and the list of materials used.
2. What is the difference between green and red antifreeze?
Pure 100% antifreeze is not used as a coolant - it is always diluted: from 20 to 35% antifreeze and 80-65% water, respectively. Only 2 types of antifreeze based on dihydric alcohols are used in heating: ethylene glycol and propylene glycol. Manufacturers produce both a concentrated composition and an already diluted one for pouring into the heating system. Ethylene glycol is a concentrated red solution, and ethylene glycol is a green solution. I will describe their differences below.
Technical characteristics of glycerin-based coolants
Glycerin based coolant
When deciding to purchase a coolant produced using glycerin, you should definitely analyze the main parameters of the latter so as not to experience unnecessary difficulties in the future with the operation and maintenance of CO:
- The temperature range in which the specified coolant will operate normally, without significant losses in its consumer parameters.
- The heat capacity of glycerin, i.e. the required amount of coolant that needs to be pumped per unit of time in order to transfer the required amount of heat.
- Viscosity coefficient, which affects the circulation rate of the coolant, the value of the heat transfer coefficient, etc. and its change depending on the temperature of the coolant.
- Corrosive activity, which imposes a number of restrictions on the use of coolant with glycerin additives without adding the required corrosion inhibitors, as well as on the choice of coolant circuit material.
- Issues of safety of using such coolants for the environment and humans.
- Lubricity, which determines the restrictions imposed by the use of the specified coolant on the design of CO elements.
- An indicator of inertness to foaming, which directly affects the efficiency of the transfer pump.